A Review on New Frontiers in Drug-Drug Interaction Predictions and Safety Evaluations with In Vitro Cellular Models
- PMID: 40574059
- PMCID: PMC12195914
- DOI: 10.3390/pharmaceutics17060747
A Review on New Frontiers in Drug-Drug Interaction Predictions and Safety Evaluations with In Vitro Cellular Models
Abstract
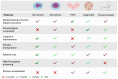
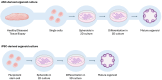
The characterization of a drug's ADME (absorption, distribution, metabolism, and excretion) profile is crucial for accurately determining its safety and efficacy. The rising prevalence of polypharmacy has significantly increased the risk of drug-drug interactions (DDIs). These interactions can lead to altered drug exposure, potentially compromising efficacy or increasing the risk of adverse drug reactions (ADRs), thereby posing significant clinical and regulatory concerns. Traditional methods for assessing potential DDIs rely heavily on in vitro models, including enzymatic assays and transporter studies. While indispensable, these approaches have inherent limitations in scalability, cost, and ability to predict complex interactions. Recent advancements in analytical technologies, particularly the development of more sophisticated cellular models and computational modeling, have paved the way for more accurate and efficient DDI assessments. Emerging methodologies, such as organoids, physiologically based pharmacokinetic (PBPK) modeling, and artificial intelligence (AI), demonstrate significant potential in this field. A powerful and increasingly adopted approach is the integration of in vitro data with in silico modeling, which can lead to better in vitro-in vivo extrapolation (IVIVE). This review provides a comprehensive overview of both conventional and novel strategies for DDI predictions, highlighting their strengths and limitations. Equipping researchers with a structured framework for selecting optimal methodologies improves safety and efficacy evaluation and regulatory decision-making and deepens the understanding of DDIs.
Keywords: cell cultures; computational modeling; cytochrome P450; drug metabolism; drug-drug interaction; in vitro to in vivo extrapolation; physiologically based pharmacokinetic model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Food and Drug Administration . Guideline for the Format and Content of the Human Pharmacokinetics and Bioavailability Section of an Application. Food and Drug Administration; Silver Spring, MD, USA: 1987.
-
- European Medicines Agency . Guideline on the Clinical Investigation of the Pharmacokinetics of Therapeutic Proteins. European Medicines Agency; London, UK: 2007.
-
- Donato M.T., Lahoz A., Castell J.V., Gómez-Lechón M.J. Cell Lines: A Tool for In Vitro Drug Metabolism Studies. Curr. Drug Metab. 2008;9:1–11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

