Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole's Antimicrobial Activity
- PMID: 40574061
- PMCID: PMC12197246
- DOI: 10.3390/pharmaceutics17060749
Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole's Antimicrobial Activity
Abstract
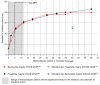
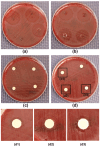
Background/Objectives: Effective drug delivery systems require precise formulation and understanding of excipient impact on active pharmaceutical ingredient (API) stability and efficacy, as uncontrolled interactions can compromise outcomes. This study developed and validated a semi-solid extrusion (SSE) 3D printing method for polyvinyl alcohol (PVA)-based hydrogel disks with metronidazole (MET). These disks served as a standardized platform to assess excipient influence on MET's antimicrobial activity, focusing on plasticizers (polyethylene glycol 400, glycerol, propylene glycol, and diethylene glycol monoethyl ether)-excipients that modify hydrogel properties for their application in printing dressing matrices-with the platform's capabilities demonstrated using in vitro antimicrobial susceptibility testing against Bacteroides fragilis. Methods: Hydrogel inks based on PVA with added plasticizers and MET were prepared. These inks were used to 3D-print standardized disks. The MET content in the disks was precisely determined. The antimicrobial activity of all formulation variants was evaluated using the disk diffusion method against B. fragilis. Results: The incorporated plasticizers did not negatively affect the antimicrobial efficacy of MET against B. fragilis. All printed hydrogel matrices exhibited clear antimicrobial activity. The 3D-printed disks showed high repeatability and precision regarding MET content. Conclusions: SSE 3D printing is viable for manufacturing precise, reproducible MET-loaded PVA hydrogel disks. It provides a standardized platform to evaluate diverse excipient impacts, like plasticizers, on API antimicrobial performance. The tested plasticizers were compatible with MET. This platform aids rational formulation design and screening for optimal excipients in designed formulations and for various pharmaceutical applications.
Keywords: 3D printing (extrusion-based); Bacteroides fragilis; antibacterial wound dressings; drug delivery; hydrogels; metronidazole; personalized therapy; plasticizers; polyvinyl alcohol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- ICH Harmonised Tripartite Guideline. Q8(R2) Pharmaceutical Development. [(accessed on 25 May 2025)]. Available online: https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf.
-
- Paul J.C., Pieper B.A. Topical Metronidazole for the Treatment of Wound Odor: A Review of the Literature. Ostomy Wound Manage. 2008;54:18–27, quiz 28–29. - PubMed
-
- Brayfield A. Martindale: The Complete Drug Reference. 38th ed. Pharmaceutical Press; London, UK: 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

