Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application
- PMID: 40574062
- PMCID: PMC12196927
- DOI: 10.3390/pharmaceutics17060750
Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application
Abstract
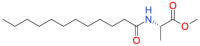
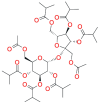
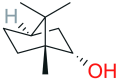
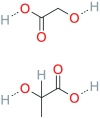
Stimuli-sensitive (in situ) drug delivery systems are a dynamically developing area of pharmaceutical research. Over the past decade, the number of studies on such systems has doubled. Among these, phase-inversion (or phase-sensitive) formulations, which were among the earliest proposed, offer significant advantages, including enhanced stability and stimuli-responsiveness. However, phase-inversion systems have remained relatively understudied. Despite the existence of three patented technologies (Atrigel®, BEPO®, FluidCrystal®) for delivery systems utilizing phase inversion for various routes of administration, the absence of unified approaches to development and standardization has significantly impeded the introduction of novel, effective drugs into clinical practice. This review examined the main polymers and solvents used to create phase-inversion compositions and discussed the feasibility of introducing other excipients to modify the systems' physicochemical properties. The most commonly used polymers included polylactide-co-glycolide, shellac, and polylactic acid. The most frequently used solvents were N-methylpyrrolidone and dimethyl sulfoxide. Following an analysis of clinical studies of phase-sensitive drugs conducted over the past 25 years, as well as original research indexed in PubMed, ScienceDirect, and Google Scholar, the main problems hindering the broader adoption of phase-inversion systems in clinical practice were identified, and recommendations for further development in this promising area were provided.
Keywords: PLGA; gels; in situ implant; phase-inversion system; solvent exchange.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

