Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections
- PMID: 40574082
- PMCID: PMC12196266
- DOI: 10.3390/pharmaceutics17060770
Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections
Abstract
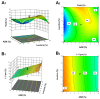
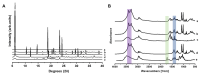
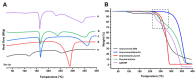
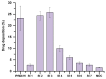
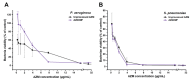
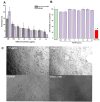
Background/Objectives: Respiratory bacterial infections remain a significant global health challenge, with effective drug delivery to the lungs being crucial for successful treatment. This study aimed to develop a lactose-free dry powder inhaler (DPI) formulation containing azithromycin (AZM) microparticles for enhanced pulmonary delivery. Methods: Using a quality-by-design approach, an optimized formulation (4% AZM, 20% leucine, and 76% mannitol) was achieved. Results: The formulation demonstrated excellent aerodynamic properties with a mass median aerodynamic diameter (MMAD) of 2.72 μm ± 0.01 μm and fine particle fraction (FPF) (<5 μm) of 65.42% ± 5.12%. AZM-loaded microparticles exhibited enhanced efficacy against Pseudomonas aeruginosa with a two-fold reduction in the minimum bactericidal concentration (7.81 μg/mL vs. 15.62 μg/mL) compared to unprocessed AZM, while maintaining activity against Streptococcus pneumoniae. AZM microparticles demonstrated good biocompatibility with red blood cells and bronchial epithelial cells at therapeutic concentrations. Conclusions: These findings establish a promising lactose-free antibiotic formulation for targeted pulmonary delivery with enhanced antimicrobial efficacy.
Keywords: azithromycin; dry powder inhaler; lactose-free; microparticles; pulmonary drug delivery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Troeger C., Blacker B., Khalil I.A., Rao P.C., Cao J., Zimsen S.R., Albertson S.B., Deshpande A., Farag T., Abebe Z. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
-
- Burke D., Harrison M., Fleming C., McCarthy M., Shortt C., Sulaiman I., Murphy D., Eustace J., Shanahan F., Hill C. Clostridium difficile carriage in adult cystic fibrosis (CF); implications for patients with CF and the potential for transmission of nosocomial infection. J. Cyst. Fibros. 2017;16:291–298. doi: 10.1016/j.jcf.2016.09.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

