Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition
- PMID: 40574095
- PMCID: PMC12196280
- DOI: 10.3390/pharmaceutics17060783
Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition
Abstract
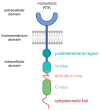
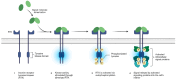
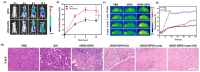
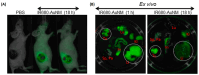
Medical research is at the forefront of addressing pressing global challenges, including preventing and treating cardiovascular, autoimmune, and oncological diseases, neurodegenerative disorders, and the growing resistance of pathogens to antibiotics. Understanding the molecular mechanisms underlying these diseases, using advanced medical approaches and cutting-edge technologies, structure-based drug design, and personalized medicine, is critical for developing effective therapies, specifically anticancer treatments. Background/Objectives: One of the key drivers of cancer at the cellular level is the abnormal activity of protein enzymes, specifically serine, threonine, or tyrosine residues, through a process known as phosphorylation. While tyrosine kinase-mediated phosphorylation constitutes a minor fraction of total cellular phosphorylation, its dysregulation is critically linked to carcinogenesis and tumor progression. Methods: Small-molecule inhibitors, such as imatinib or erlotinib, are designed to halt this process, restoring cellular equilibrium and offering targeted therapeutic approaches. However, challenges persist, including frequent drug resistance and severe side effects associated with these therapies. Nanomedicine offers a transformative potential to overcome these limitations. Results: By leveraging the unique properties of nanomaterials, it is possible to achieve precise drug delivery, enhance accumulation at target sites, and improve therapeutic efficacy. Examples include nanoparticle-based delivery systems for TKIs and the combination of nanomaterials with photothermal or photodynamic therapies to enhance treatment effectiveness. Combining nanomedicine with traditional treatments holds promise and perspective for synergistic and more effective cancer management. Conclusions: This review delves into recent advances in understanding tyrosine kinase activity, the mechanisms of their inhibition, and the innovative integration of nanomedicine to revolutionize cancer treatment strategies.
Keywords: anticancer treatment; drug delivery; gold nanoparticles; metal nanoparticles; nanomaterials; nanomedicine; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

