Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks
- PMID: 40574103
- PMCID: PMC12196511
- DOI: 10.3390/pharmaceutics17060791
Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks
Abstract
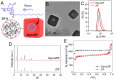
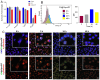
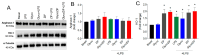
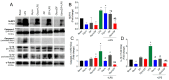
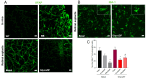
Background/Objectives: Diabetic retinopathy is an ocular disease caused by changes in the expression of inflammatory mediators and increased oxidative stress in the retina and is the leading cause of vision loss in diabetic patients. Currently, there is no treatment capable of reversing retinal damage, which represents a significant burden on the quality of life of patients. (1R)-1-Dodecylsulfonyl-5N,6O-oxomethylidenenojirimycin stands outs as a prototype of the sp2-iminoglycolipids family for its beneficial neuroprotective effect against this chronic eye disease. Critical issues related to the low solubility and bioavailability of this glycolipid in biological settings are overcome by its encapsulation in a Zeolitic-Imidazolate Framework (ZIF) structure, resulting in homogeneous and biocompatible GlycoZIF nanoparticles. Cell studies show an enhanced cellular uptake compared with the free glycolipid, and importantly, its bioactivity is preserved once released inside cells. Methods: Extensive in vitro and ex vivo assays with diabetic retinopathy models unveil the mechanistic pathways of the designed GlycoZIF. Results: A reduction in proinflammatory mediators, increased heme oxygenase-1 level, inhibition of NLRP3 inflammasome, and reduced reactive gliosis is shown. Conclusions: These findings demonstrate for the first time the potential of Glyco-modified ZIFs for the treatment of diabetes-related ocular problems by controlling the immune-mediated inflammatory response.
Keywords: diabetic retinopathy; glycolipid; immune-mediated therapy; inflammation; microglia; zeolitic-imidazolate framework.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ogurtsova K., Da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. - DOI - PubMed
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

