Advancements in Catalytic Depolymerization Technologies
- PMID: 40574142
- PMCID: PMC12197214
- DOI: 10.3390/polym17121614
Advancements in Catalytic Depolymerization Technologies
Abstract
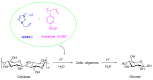
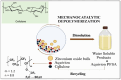
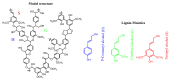
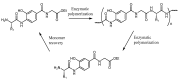
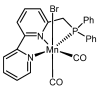
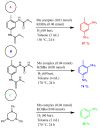
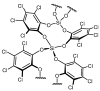
The increasing market demand and rising costs of raw materials have intensified interest in renewable and sustainable sources. As a result, the production of building-block chemicals from natural products or synthetic feedstocks has driven scientific research toward catalytic strategies for the depolymerization of these materials. Polymer chemistry offers significant opportunities for recycling, as polymer synthesis typically begins with monomeric units. Emerging non-destructive techniques now allow for the recovery of these original reagents. This review summarizes recent advances in catalytic methods for the depolymerization of polymers derived from both natural sources, such as cellulose and lignin, and synthetic sources, including conventional plastics. The review is structured in three main sections: catalytic depolymerization of cellulose, lignin, and plastics. Special emphasis is placed on recent studies that explore innovative methodologies. The raw materials obtained through these processes can be reintegrated into production cycles, contributing to the development of a fully circular economy.
Keywords: catalysis; circular economy; polymers; sustainable chemistry.
Conflict of interest statement
Author Pravin Jagdale was employed by the company BHUMI–Bharat Harit Urja Management and Innovations Pvt Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
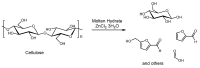




References
-
- Kreps B.H. The Rising Costs of Fossil-Fuel Extraction: An Energy Crisis That Will Not Go Away. Am. J. Econ. Sociol. 2020;79:695–717. doi: 10.1111/ajes.12336. - DOI
-
- Ghazouani T., Maktouf S. Impact of natural resources, trade openness, and economic growth on CO2 emissions in oil-exporting countries: A panel autoregressive distributed lag analysis. Nat. Resour. Forum. 2024;48:211–231. doi: 10.1111/1477-8947.12318. - DOI
-
- Zibunas C., Meys R., Kätelhön A., Bardow A. Cost-optimal pathways towards net-zero chemicals and plastics based on a circular carbon economy. Comput. Chem. Eng. 2022;162:107798. doi: 10.1016/j.compchemeng.2022.107798. - DOI
-
- Boulamanti A., Moya J.A. Production costs of the chemical industry in the EU and other countries: Ammonia, methanol and light olefins. Renew. Sustain. Energy Rev. 2017;68:1205–1212. doi: 10.1016/j.rser.2016.02.021. - DOI
-
- Axon S., James D. The UN Sustainable Development Goals: How can sustainable chemistry contribute? A view from the chemical industry. Curr. Opin. Green Sustain. Chem. 2018;13:140–145. doi: 10.1016/j.cogsc.2018.04.010. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

