Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers
- PMID: 40574216
- PMCID: PMC12197038
- DOI: 10.3390/polym17121688
Encapsulation of Perfluoroalkyl Carboxylic Acids (PFCAs) Within Polymer Microspheres for Storage in Supercritical Carbon Dioxide: A Strategy Using Dispersion Polymerization of PFCA-Loaded Monomers
Abstract
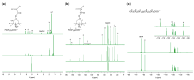
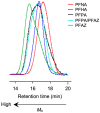
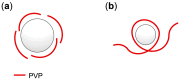
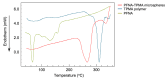
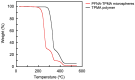
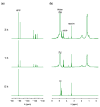
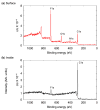
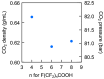
The removal of per- and polyfluoroalkyl substances (PFAS) from global aquatic environments is an emerging issue. However, little attention has been paid to addressing accumulated PFAS through their removal. This study demonstrates the encapsulation of perfluoroalkyl carboxylic acids (PFCAs) within polymer microspheres that dissolve in supercritical carbon dioxide (scCO2). PFCAs were effectively captured by a hindered amine-supported monomer, 2,2,6,6-tetramethyl-4-piperidyl methacrylate (TPMA), in methanol (MeOH) through a simple acid-base reaction. The PFCA-loaded TPMA underwent dispersion polymerization in MeOH in the presence of poly(N-vinylpyrrolidone) (PVP) as a surfactant, producing microspheres with high monomer conversions. The microsphere size depended on the molecular weight and concentration of PVP, as well as the perfluoroalkyl chain length of the PFCAs. X-ray photoelectron spectroscopy (XPS) revealed that the perfluoroalkyl chains migrated from the interior to the surface of the microspheres when exposed to air. These surface perfluoroalkyl chains facilitated dissolution of the microspheres in scCO2, with cloud points observed under relatively mild conditions. These findings suggest the potential for managing PFCA-encapsulated microspheres in the scCO2 phase deep underground via CO2 sequestration.
Keywords: PFCA-loaded monomer; acid-base reaction; cloud point; dispersion polymerization; electrostatic crosslinking; encapsulation; microspheres; perfluoroalkyl carboxylic acids (PFCAs); perfluoroalkyl chains; supercritical carbon dioxide.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
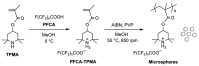


Similar articles
-
Chain length-dependent mitochondrial toxicity of perfluoroalkyl carboxylic acids: insights from Mito Tox Index evaluation.Front Toxicol. 2025 Jul 15;7:1582891. doi: 10.3389/ftox.2025.1582891. eCollection 2025. Front Toxicol. 2025. PMID: 40734953 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
pH-Responsive Diblock Copolymer Vesicles via Polymerization-Induced Self-Assembly in Aqueous Media: Synthesis, Loading, and Potential Biological Applications.ACS Appl Mater Interfaces. 2025 Jun 18;17(24):35140-35154. doi: 10.1021/acsami.5c05091. Epub 2025 Jun 3. ACS Appl Mater Interfaces. 2025. PMID: 40460265
-
Defluorination of per- and polyfluoroalkyl carboxylic acids (PFCAs) by wood decomposer fungi.Mycologia. 2025 Jul-Aug;117(4):576-588. doi: 10.1080/00275514.2025.2499476. Epub 2025 Jun 3. Mycologia. 2025. PMID: 40459903
References
-
- Kancharla S., Jahan R., Bedrov D., Tsianou M., Alexandridis P. Role of chain length and electrolyte on the micellization of anionic fluorinated surfactants in water. Colloids Surf. A. 2021;628:127313. doi: 10.1016/j.colsurfa.2021.127313. - DOI
-
- Itumoh E.J., Data S., Chen J.L.-Y., Kah M., Padhye L.P., Leitao E.M. Addressing the persistence of per and poly- fluoroalkyl substances (PFAS): Current challenges and potential solutions. RSC Sustain. 2024;2:3183–3201. doi: 10.1039/D4SU00152D. - DOI
-
- Grunfeld D.A., Gilbert D., Hou J., Jones A.M., Lee M.J., Tohren C.G., Kibbey T.C.G., O’Carroll D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024;17:340–346. doi: 10.1038/s41561-024-01402-8. - DOI
-
- Kurwadkar S., Dane J., Kanel S.R., Nadagouda M.N., Cawdrey R.W., Ambade B., Struckhoff G.C., Wilkin R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022;809:151003. doi: 10.1016/j.scitotenv.2021.151003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

