The role of VISTA engagement in limiting neutrophil-mediated inflammation
- PMID: 40574311
- PMCID: PMC12336919
- DOI: 10.1093/jimmun/vkaf132
The role of VISTA engagement in limiting neutrophil-mediated inflammation
Abstract
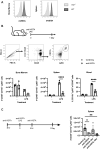
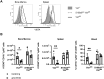
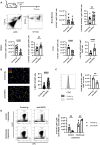
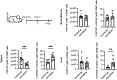
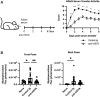
A growing body of evidence suggests that the immune checkpoint inhibitory receptor VISTA plays a central role in the regulation of innate immunity in the settings of inflammatory diseases and cancer. Neutrophils are among the cells that have the highest membrane density of surface VISTA. In this study, targeting VISTA on neutrophils with a monoclonal antibody resulted in a striking reduction in their lipopolysaccharide (LPS)- and chemokine-induced peripheral accumulation but did not reduce neutrophil levels at steady state. Fc receptor engagement and macrophages were required for the effects of anti-VISTA antibody on neutrophils. Concomitant with reduced peripheral neutrophil numbers, targeting VISTA increased neutrophil clearance by macrophages in the liver. In a murine model of neutrophil-mediated arthritis, anti-VISTA antibody treatment ameliorated disease severity, which was associated with reduced myeloperoxidase activity in the joints. These studies identify a novel therapeutic opportunity for targeting VISTA to control neutrophil-mediated inflammation and tissue injury.
Keywords: VISTA; immune checkpoint; inflammation; neutrophils.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
None declared.
Figures

References
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. - PubMed
-
- Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. - PubMed
-
- Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI148430/AI/NIAID NIH HHS/United States
- R21 AR079661/AR/NIAMS NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- R01 HL155114/HL/NHLBI NIH HHS/United States
- R21 AR079661-01/AR/NIAMS NIH HHS/United States
- R01 AI148430/AI/NIAID NIH HHS/United States
- R21 AR079661-01/AR/NIAMS NIH HHS/United States
- R01 HL155114/HL/NHLBI NIH HHS/United States
- 2022 Lupus Research Alliance Lupus Innovation Award
- W81XWH2110889/Congressionally Directed Medical Research Programs Lupus Research Program
- Dartmouth's Immune Monitoring and Flow Cytometry Resource
- NIH
- 5P30CA023108/National Cancer Institute Cancer
LinkOut - more resources
Full Text Sources
Research Materials

