Mass Spectral Analysis of Sterols and Other Steroids in Different Ionization Modes: Sensitivity and Oxidation Artifacts
- PMID: 40574312
- PMCID: PMC12333350
- DOI: 10.1021/jasms.5c00099
Mass Spectral Analysis of Sterols and Other Steroids in Different Ionization Modes: Sensitivity and Oxidation Artifacts
Abstract
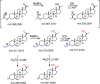
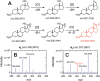
In the course of synthetic work and mass spectrometry (MS) with hydroxy steroids, we observed not only the loss of H2O but prominent 2 and 4 amu losses using atmospheric pressure chemical ionization (APCI), leading to confusion of the structural assignments. This loss of 2 amu, which we attributed mainly to oxidation of hydroxyls, varied among 44 steroids and sterols analyzed; 36 showed losses of 2n amu in APCI MS analysis (17/22 Δ5 steroids, 17/19 Δ4 steroids, and 2/3 estrogens). With the Δ5 steroids and sterols, the precursor MH+ was either observed as a minor ion or (more frequently) not detected at all, constituting the base peak in 7/22 cases. With heated electrospray ionization (HESI) MS, 2n amu losses were detected (generally weakly) in 9/44 cases but constituted the base peak in 3/9. In general, the sensitivity (base peak intensity) of steroids correlated with conjugation of the of the steroid frame. Δ4 steroids generally performed best on HESI+ (up to a maximum factor of 8-fold), while Δ5 steroids and sterols performed better on APCI+ (up to >137-fold), except for two trihydroxypregnanes. Estrogens did not show a clear trend. Sensitivity generally increased with the use of NH4F as a mobile phase additive in ESI+ (up to a maximum of 7-fold). We conclude that the prominence of 2n amu losses is variable among steroids and sterols but is more commonly an artifact of APCI MS. These m/z losses can constitute dominant ions that impede detection of the precursor MH+ and complicate structural assignments.
Keywords: APCI; HESI; ammonium fluoride; artifacts; hydroxy steroids; mass spectrometry; oxidation; steroids.
Figures




Similar articles
-
Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery.Cochrane Database Syst Rev. 2016 Nov 1;11(11):CD006683. doi: 10.1002/14651858.CD006683.pub3. Cochrane Database Syst Rev. 2016. PMID: 27801522 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Topical antibiotics with steroids for chronic suppurative otitis media.Cochrane Database Syst Rev. 2025 Jun 9;6(6):CD013054. doi: 10.1002/14651858.CD013054.pub3. Cochrane Database Syst Rev. 2025. PMID: 40484406
-
Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children.Cochrane Database Syst Rev. 2013 Dec 16;2013(12):CD009019. doi: 10.1002/14651858.CD009019.pub2. Cochrane Database Syst Rev. 2013. PMID: 24343671 Free PMC article.
-
Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure.Cochrane Database Syst Rev. 2017 Feb 22;2(2):CD012131. doi: 10.1002/14651858.CD012131.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 6;6:CD012131. doi: 10.1002/14651858.CD012131.pub3. PMID: 28225198 Free PMC article. Updated.
References
-
- Budzikiewicz H., Djerassi C.. Mass Spectrometry in Structural and Stereochemical Problems. I. Steroid Ketones. J. Am. Chem. Soc. 1962;84(8):1430–1439. doi: 10.1021/ja00867a019. - DOI
-
- Mitsui K., Ochiai N., Higashi N., Dunkle M., Sasamoto K., Tienpont B., David F., Sandra P.. Gas Chromatography with Soft Ionization Mass Spectrometry for the Characterization of Natural Products. LCGC Eur. 2013;26(10):548–546.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

