Astrocytic abnormalities in brain-specific Cacna1c-deficient mice: Implications for BBB impairment in neuropsychiatric diseases associated with CACNA1C mutations
- PMID: 40574410
- PMCID: PMC12218471
- DOI: 10.1080/19336950.2025.2523788
Astrocytic abnormalities in brain-specific Cacna1c-deficient mice: Implications for BBB impairment in neuropsychiatric diseases associated with CACNA1C mutations
Abstract
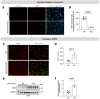
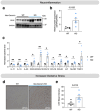
Intronic genetic variants within the CACNA1C gene, which encodes the pore-forming alpha 1c subunit of the Cav1.2 L-type calcium channel, are significant risk factors for a multitude of neuropsychiatric disorders. In most cases, these intronic SNPs have been associated with reduced CACNA1C expression. Here, we demonstrate that targeted genetic deletion of Cacna1c in mouse brain leads to increased astrocyte reactivity, increased expression of aquaporin 4 (AQP4) in astrocytes adjacent to the blood-brain barrier (BBB), and neuroinflammation, including changes in the levels of brain chemokines and inflammatory cytokines. Astrocytes are vital for maintaining BBB integrity, with AQP4 predominantly expressed in astrocytic endfeet where it regulates water balance in the brain. This function is critical to brain health, and deterioration of the BBB is a major feature of virtually all forms of neuropsychiatric disease. Our results highlight a previously unrecognized role for CACNA1C in astrocytes at the BBB, which could be a major factor in how intronic CACNA1C SNPs broadly increase the risk of multiple forms of major neuropsychiatric disease.
Keywords: AQP4; CACNA1C; Calcium; Cav1.2; aquaporin 4; neuropsychiatric disease.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Reduced Gene Dosage of the Psychiatric Risk Gene Cacna1c Is Associated with Impairments in Hypothalamic-Pituitary-Adrenal Axis Activity in Rats.Int J Mol Sci. 2025 Jun 10;26(12):5547. doi: 10.3390/ijms26125547. Int J Mol Sci. 2025. PMID: 40565009 Free PMC article.
-
SOAT1 dysregulation in astrocytes drives Blood-Brain barrier dysfunction and neuroinflammation in Alzheimer's disease.Brain Behav Immun. 2025 Aug;128:497-509. doi: 10.1016/j.bbi.2025.04.032. Epub 2025 Apr 22. Brain Behav Immun. 2025. PMID: 40274003
-
Crucial role of Aquaporin-4 extended isoform in brain water Homeostasis and Amyloid-β clearance: implications for Edema and neurodegenerative diseases.Acta Neuropathol Commun. 2024 Oct 10;12(1):159. doi: 10.1186/s40478-024-01870-4. Acta Neuropathol Commun. 2024. PMID: 39385254 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
