Guselkumab in East Asians With Moderate-to-Severe Ulcerative Colitis: Subgroup Analysis of the QUASAR Induction and Maintenance Studies
- PMID: 40574492
- PMCID: PMC12400247
- DOI: 10.1111/jgh.17036
Guselkumab in East Asians With Moderate-to-Severe Ulcerative Colitis: Subgroup Analysis of the QUASAR Induction and Maintenance Studies
Abstract
Background and aim: The global QUASAR (NCT04033445) clinical program demonstrated the efficacy and safety of guselkumab, a dual-acting interleukin-23 p19 subunit inhibitor, as induction and maintenance therapy in participants with moderate to severely active ulcerative colitis (UC). We report a subgroup analysis in East Asian participants.
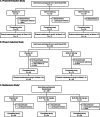
Methods: The QUASAR program included two randomized, placebo-controlled, 12-week induction studies of guselkumab 200 mg (and 400 mg, Phase 2b) IV every 4 weeks (q4w) in adults with baseline modified Mayo scores of 5-9 and inadequate response/intolerance to conventional and/or advanced UC therapy. Clinical responders to guselkumab induction were re-randomized (1:1:1) at maintenance study baseline to SC guselkumab 200 mg q4w, 100 mg q8w, or placebo. Primary endpoints were clinical response (Phase 2b) or clinical remission (Phase 3) at induction Week 12 (I-12) and clinical remission at maintenance Week 44 (M-44). Subgroup analyses included participants from sites in China, Japan, Korea, and Taiwan region.
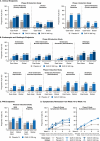
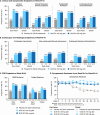
Results: Data were from 71 (Phase 2b) and 135 (Phase 3) East Asians in the induction studies and 106 in the maintenance study. At Week I-12, 45.5%-58.8% of guselkumab versus 25.5%-29.2% of placebo participants achieved clinical response and 16.0%-23.8% versus 4.2%-5.5%, respectively, achieved clinical remission. At Week M-44, 37.1%-46.3% of guselkumab versus 13.3% of placebo participants achieved clinical remission. The adverse event profile was generally consistent with the global QUASAR population.
Conclusions: Results support the efficacy and safety of guselkumab induction and maintenance in East Asians with moderately to severely active UC, consistent with findings from the global QUASAR studies.
Trial registration: ClinicalTrials.gov, NCT04033445; EudraCT, 2018-004002-25.
Keywords: Asia; clinical trials; guselkumab; ulcerative colitis.
© 2025 Johnson & Johnson and The Author(s). Journal of Gastroenterology and Hepatology published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Baili Chen has nothing to disclose.
Byong Duk Ye is an Editorial Board member of
Qian Cao served as a steering committee advisor for Bristol Myers Squibb Company and Janssen Research Development LLC and has received research grants from Johnson & Johnson and Takeda.
Fumihito Hirai has nothing to disclose.
Masayuki Saruta has received grants or contracts from AbbVie GK, CMIC CMO Co. Ltd., Kissei Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., PPD‐SNBL K.K., and Zeria Pharmaceutical Co. Ltd.; and payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from AbbVie GK, EA Pharma Co. Ltd., Gilead Sciences K.K., Janssen Pharmaceutical K.K., Kissei Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., Nobelpharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Viatris Pharmaceutical Co. Ltd.
Minhu Chen received research grants and served as a steering committee advisor for Johnson & Johnson Company, and received lecture fees from Johnson & Johnson, Takeda, AbbVie, and China Medical System Holding Limited.
Susan Pelak, Nicole Shipitofsky, Ye Miao, Keira Herr, Bryan Wahking, and Jianmin Zhuo are employees of Johnson & Johnson and may own company stock/stock options.
Tadakazu Hisamatsu reports grant support from AbbVie GK, Boston Scientific Corporation, EA Pharma Co. Ltd., JIMRO Co. Ltd., Kissei Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Pfizer Inc., Takeda Pharmaceutical Co. Ltd., and Zeria Pharmaceutical Co. Ltd.; consulting fees from AbbVie GK, Bristol Myers Squibb, EA Pharma Co. Ltd., Eli Lilly, Gilead Sciences, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, and Pfizer Inc.; and lecture fees from AbbVie GK, EA Pharma Co. Ltd., Janssen Pharmaceutical K.K., JIMRO Co., Kissei Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., Pfizer Inc., and Takeda Pharmaceutical Co. Ltd.
Figures

References
-
- Feuerstein J. D., Moss A. C., and Farraye F. A., “Ulcerative Colitis,” Mayo Clinic Proceedings 94, no. 7 (2019): 1357–1373. - PubMed
-
- Kuo C. J., Lin C. Y., Le P. H., et al., “Temporal Trends of Inflammatory Bowel Diseases in Taiwan From 2016 to 2020: A Population‐Based Study,” Digestive Diseases and Sciences 69, no. 9 (2024): 3172–3179. - PubMed
-
- Mak W. Y., Zhao M., Ng S. C., and Burisch J., “The Epidemiology of Inflammatory Bowel Disease: East Meets West,” Journal of Gastroenterology and Hepatology 35, no. 3 (2020): 380–389. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

