Improvements in medical therapy and prognosis for patients with HFrEF following the 2021 ESC HF guidelines
- PMID: 40574622
- PMCID: PMC12450798
- DOI: 10.1002/ehf2.15337
Improvements in medical therapy and prognosis for patients with HFrEF following the 2021 ESC HF guidelines
Abstract
Aims: The guideline-directed medical therapy (GDMT) sequencing strategy for patients with heart failure (HF) and reduced ejection fraction (HFrEF) underwent a paradigm shift with the 2021 ESC HF guidelines, from stepwise escalation to rapid simultaneous initiation of quadruple therapy. We aimed to assess the temporal trends in the use of GDMT and prognosis for patients with HFrEF.
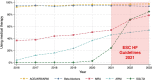
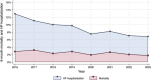
Methods and results: Through the Norwegian HF Registry, we obtained data on patients treated at HF outpatient clinics with left ventricular ejection fraction ≤40% from 2016 through 2023 (n = 13 992), including GDMT, HF hospitalisations and mortality. Since 2016, >90% of patients have been treated with beta-blockers and renin-angiotensin-system-inhibitors, with angiotensin receptor-neprilysin inhibitors (ARNI) utilisation increasing from 4% in 2016 to 54% in 2023. Mineralocorticoid-receptor-antagonists (MRA) utilisation was at 36% in 2016, increased by 3% per year to 54% in 2021, and thereafter increased by 12% per year to 78% in 2023. Sodium-glucose cotransporter-2-inhibitors (SGLT2i) utilisation increased rapidly from 3% in 2020 to 85% in 2023. The utilisation of ≥50% of target dose followed similar trends. From 2016 to 2021, the crude 6-month mortality rate remained at 2.7%, followed by a decline of approximately 0.5% per year to 1.8% in 2023. HF hospitalisations declined steadily from 12.9% in 2016 to 8.2% in 2021, with a further decline to 6.8% in 2023.
Conclusions: The utilisation of GDMT in Norwegian HF clinics has increased markedly since 2016, with a fourfold acceleration in MRA and a substantial increase in SGLT2i use following the 2021 ESC HF guidelines. HF hospitalisations have consistently declined, while mortality rates first declined after 2021.
Keywords: Guideline‐directed medical therapy; HFrEF; Heart failure; Outcomes; Temporal trends.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
KB has received speaker fees from Boehringer‐Ingelheim, Novartis, Amgen, and AstraZeneca. HS has received speaker fees from Amgen, AstraZeneca, Biogen, Boehringer‐Ingelheim, Novartis, Novo Nordisk, and Pharmacosmos. LG received speaker fees from AstraZeneca, Bayer, Boehringer‐Ingelheim, Novartis, and Novo Nordisk. TO reports receiving honoraria from Abbott Diagnostics, Bayer, CardiNor, NovoNordisk, and Roche Diagnostics, and research support from Abbott Diagnostics, CardiNor, ChromaDex, Novartis and Roche Diagnostics, via Akershus University Hospital. KL has received speakers fee and/or has served on advisory board for Boehringer‐Ingelheim, Bayer, Astra Zeneca, Orion Pharma, Novartis, Vifor, Roche and Novo Nordisk. RM has served on advisory boards and/or received speaker fees from Astra Zeneca, Bayer, Boehringer‐Ingelheim, Novartis and Pfizer. SØ has served on advisory boards and/or received speaker fees from AstraZeneca, Pfizer, Bayer, Boehringer‐Ingelheim, Novartis, Novo Nordisk, Sanofi, MSD, and Philips. PLM has served on advisory boards and/or received speaker fees from Amarin, AmGen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol Myers Squibb, Novartis, Novo Nordisk, Pharmacosmos, Roche, Sanofi, US2.ai and Vifor.
Figures
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18:891‐975. doi: 10.1002/ejhf.592 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous