Structural dynamics of IDR interactions in human SFPQ and implications for liquid-liquid phase separation
- PMID: 40574713
- PMCID: PMC12216677
- DOI: 10.1107/S2059798325005303
Structural dynamics of IDR interactions in human SFPQ and implications for liquid-liquid phase separation
Abstract
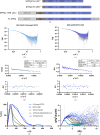
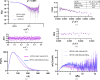
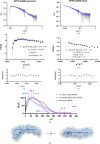
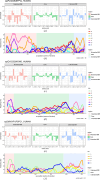
The proteins SFPQ (splicing factor proline- and glutamine-rich) and NONO (non-POU domain-containing octamer-binding protein) are members of the Drosophila behaviour/human splicing (DBHS) protein family, sharing 76% sequence identity in their conserved DBHS domain. These proteins are critical for elements of pre- and post-transcriptional regulation in mammals and are primarily located in paraspeckles: ribonucleoprotein bodies templated by NEAT1 long noncoding RNA. Regions that are structured and predicted to be disordered (IDRs) in DBHS proteins facilitate various interactions, including dimerization, polymerization, nucleic acid binding and liquid-liquid phase separation, all of which have consequences for cell health, the pathology of some neurological diseases and cancer. To date, very limited structural work has been carried out on characterizing the IDRs of the DBHS proteins, largely due to their predicted disordered nature and the fact that this is often a bottleneck for conventional structural techniques. This is a problem worth addressing, as the IDRs have been shown to be critical to the material state of the protein as well as its function. In this study, we used small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS), together with lysine cross-linking mass spectrometry (XL-MS), to investigate the regions of SFPQ flanking the structured DBHS domain and the possibility of dimer partner exchange of full-length proteins. Our results demonstrate experimentally that the N- and C-terminal regions on either side of the folded DBHS domain are long, disordered and flexible in solution. Realistic modelling of disordered chains to fit the scattering data and the compaction of the different protein variants suggests that it is physically possible for the IDRs to be close enough to interact. The mass-spectrometry data additionally indicate that the C-terminal IDR can potentially interact with the folded DBHS domain and also shares some conformational space with the N-terminal IDR. Our small-angle neutron scattering (SANS) experiments reveal that full-length SFPQ is capable of swapping dimer partners with itself, which has implications for our understanding of the combinatorial dimerization of DBHS proteins within cells. Our study provides insight into possible interactions between different IDRs either in cis or in trans and how these may relate to protein function, and the possible impact of mutations in these regions. The dynamic dimer partner exchange of a full-length protein inferred from this study is a phenomenon that is integral to the function of DBHS proteins, allowing changes in gene-regulatory activity by altering levels of the various heterodimers or homodimers.
Keywords: DBHS; dimers; disorder; flexibility; phase separation.
open access.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

