Functional remapping in networks of the Parkinsonian brain: A preclinical neuroimaging perspective with clinical correlates
- PMID: 40574753
- PMCID: PMC12198951
- DOI: 10.1515/tnsci-2025-0374
Functional remapping in networks of the Parkinsonian brain: A preclinical neuroimaging perspective with clinical correlates
Abstract
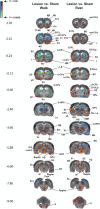
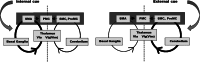
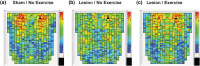
Parkinson's disease (PD) is increasingly understood as a neurodegenerative condition whose pathology extends beyond the direct and indirect basal ganglia pathways. Clinically, patients are all too painfully aware of dysfunction not only of motor circuits but also of somatosensory, autonomic, cognitive, and emotional systems. Functional neuroimaging studies have begun to document a functional reorganization in the PD brain across a wide number of networks. In particular, the cerebellar-thalamocortical, as well as the fronto-striatal circuit, have been shown to undergo functional reorganization. In this narrative review, citing preclinical as well as clinical neuroimaging studies, our objective is to highlight trends and discuss the relevance of cerebral adaptive changes. It remains clear that not all changes contribute to the normalization of functions. Parsing differences between functional "compensation," "silencing," or "maladaptation" in neural circuits is important. A necessary next step in neurorehabilitation is the question of whether compensatory cerebral changes can be enhanced. In this regard, physical exercise remains of interest, given that in patients, exercise may allow some degree of symptom improvement and possibly slow the course of the disease. Future interventions may wish to integrate neuroimaging findings as potential targets to support neuroplastic changes.
Keywords: Parkinson’s disease; exercise; functional brain mapping; neurorehabilitation; plasticity.
© 2025 the author(s), published by De Gruyter.
Conflict of interest statement
Conflict of interest: The authors state no conflict of interest.
Figures

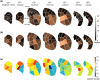

Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Physical exercise for people with Parkinson's disease: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2023 Jan 5;1(1):CD013856. doi: 10.1002/14651858.CD013856.pub2. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2024 Apr 08;4:CD013856. doi: 10.1002/14651858.CD013856.pub3. PMID: 36602886 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
-
Differently different?: A commentary on the emerging social cognitive neuroscience of female autism.Biol Sex Differ. 2024 Jun 13;15(1):49. doi: 10.1186/s13293-024-00621-3. Biol Sex Differ. 2024. PMID: 38872228 Free PMC article. Review.
References
-
- Mountz JM. Nuclear medicine in the rehabilitative treatment evaluation in stroke recovery. Role of diaschisis resolution and cerebral reorganization. Eura Medicophys. 2007;43(2):221–39. - PubMed
-
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: How relevant is the rat? Nat Rev Neurosci. 2002;3(7):574–9. - PubMed
-
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76(5):1265–74. - PubMed
-
- Jahn K, Deutschlander A, Stephan T, Kalla R, Hufner K, Wagner J, et al. Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res. 2008;171:353–62. - PubMed
-
- Paxinos G, Watson C. The rat brain in stereotactic coordinates. 6th edn. New York: Elsevier Academic Press; 2007.
Publication types
LinkOut - more resources
Full Text Sources
