The gut-immune axis in primary immune thrombocytopenia (ITP): a paradigm shifts in treatment approaches
- PMID: 40574831
- PMCID: PMC12198146
- DOI: 10.3389/fimmu.2025.1595977
The gut-immune axis in primary immune thrombocytopenia (ITP): a paradigm shifts in treatment approaches
Abstract
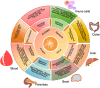
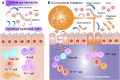
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by platelet destruction and impaired production, leading to bleeding risk. While immunosuppressive therapies are standard, many patients experience relapses or refractory disease, highlighting the need for novel approaches. Emerging evidence suggests the gut microbiota plays a role in immune regulation, yet its impact on ITP remains unclear. Dysbiosis has been linked to immune dysfunction in other autoimmune diseases, but whether it drives or results from immune dysregulation in ITP is debated. This review explores the gut-immune axis in ITP, focusing on microbiota-driven immune modulation, cytokine signaling, and platelet homeostasis. We assess microbiota-targeted interventions, including fecal microbiota transplantation (FMT), probiotics, and dietary modifications, while addressing key controversies and knowledge gaps. Advances in microbiome sequencing and artificial intelligence may facilitate personalized interventions. Standardizing microbiota-based diagnostics and validating their efficacy in clinical trials are crucial for their integration into ITP management. Bridging these gaps may lead to microbiota-driven strategies that enhance immune regulation and improve patient outcomes.
Keywords: gut-immune axis; immune dysregulation; microbiota-based therapy; platelet homeostasis; primary immune thrombocytopenia (ITP).
Copyright © 2025 Guo, Wang, Liu, Baran and Ma.
Conflict of interest statement
WM is a scientific and medical advisor at Calidi Biotherapeutics Inc. San Diego, CA, USA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. WM holds the position of associate editor of Frontiers in Immunology at the time of submission. This had no impact on the peer review process or the final decision.
Figures

Similar articles
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Gut modulation to regulate NF-κB in colorectal and gastric cancer therapy and inflammation.Cancer Immunol Immunother. 2025 Jul 12;74(8):264. doi: 10.1007/s00262-025-04118-9. Cancer Immunol Immunother. 2025. PMID: 40650758 Free PMC article. Review.
-
Gut microbiome composition and dysbiosis in immune thrombocytopenia: A review of literature.Blood Rev. 2024 Sep;67:101219. doi: 10.1016/j.blre.2024.101219. Epub 2024 Jun 6. Blood Rev. 2024. PMID: 38862311 Review.
-
Gut microbiota-driven neuroinflammation in Alzheimer's disease: from mechanisms to therapeutic opportunities.Front Immunol. 2025 Jun 26;16:1582119. doi: 10.3389/fimmu.2025.1582119. eCollection 2025. Front Immunol. 2025. PMID: 40642089 Free PMC article. Review.
-
Fecal microbiota transplantation for the management of autoimmune diseases: Potential mechanisms and challenges.J Autoimmun. 2023 Dec;141:103109. doi: 10.1016/j.jaut.2023.103109. Epub 2023 Sep 9. J Autoimmun. 2023. PMID: 37690971
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

