Long-term immune checkpoint inhibitor therapy in a patient with metastatic nasopharyngeal carcinoma: a case report
- PMID: 40574835
- PMCID: PMC12198176
- DOI: 10.3389/fimmu.2025.1585844
Long-term immune checkpoint inhibitor therapy in a patient with metastatic nasopharyngeal carcinoma: a case report
Abstract
Background: Immunotherapy has revolutionized cancer treatment. However, the duration of treatment and the timing of discontinuation are major concerns. Current pivotal trials predominantly advocate for a fixed two-year regimen of immune checkpoint inhibitors (ICIs), exemplified by pembrolizumab and toripalimab, as first-line therapy for patients with advanced malignancies. Alternatively, for specific ICIs, including nivolumab, camrelizumab, and tislelizumab, continuous administration until disease progression has emerged as a favored approach. Nevertheless, whether to discontinue treatment after two years remains intensely debated within the medical community, underscoring the need for further research to clarify optimal treatment durations.
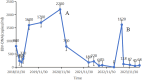
Case presentation: In November 2018, a 44-year-old male presented with a persistent headache. Following a positive nasopharyngeal mucosal biopsy, he was diagnosed with non-keratinizing undifferentiated carcinoma of the nasopharynx cT4N2M0. An Epstein-Barr Virus (EBV) DNA load of 800 copies/mL was detected. The patient completed two cycles of induction chemotherapy with liposomal paclitaxel and nedaplatin, followed by platinum-based concurrent chemoradiotherapy, resulting in a progression-free survival (PFS) of 23.6 months. The EBV DNA load dropped significantly to 190 copies/mL. However, during a routine examination in January 2021, metastases in the lung and mediastinal lymph nodes were detected, and the EBV DNA load was measured at 2200 copies/mL. Consequently, surgical intervention was performed, followed by radiotherapy and two years of ICI treatment. Throughout the ICI maintenance period, the EBV DNA level remained consistently below the limit of detection. Remarkably, three months after treatment discontinuation, the patient exhibited a rebound in EBV DNA (1620 copies/mL). Nevertheless, imaging scans revealed no evidence of tumor progression. Following an ICI rechallenge, the patient's EBV DNA load returned to undetectable levels. The patient continues the ICI therapy and has thus far achieved a PFS of 41.6 months.
Conclusion: EBV DNA levels could serve as an informative marker to predict the necessity of therapy discontinuation during immunotherapy maintenance. Notably, a post-discontinuation ICI rechallenge can still yield favorable outcomes potentially accredited to immune memory.
Keywords: EBV DNA; immune checkpoint inhibitor; immunotherapy; therapy discontinuation; treatment duration.
Copyright © 2025 Qing, Lu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Immunotherapy combined with chemotherapy without locoregional radiotherapy in de novo metastatic nasopharyngeal carcinoma: two case reports and a literature review.Front Immunol. 2025 Jan 9;15:1467355. doi: 10.3389/fimmu.2024.1467355. eCollection 2024. Front Immunol. 2025. PMID: 39850881 Free PMC article. Review.
-
The efficacy of immunotherapy combined with capecitabine versus immunotherapy alone as maintenance therapy in patients with de novo metastatic nasopharyngeal carcinoma: a retrospective propensity score matching study.ESMO Open. 2025 Jun;10(6):105295. doi: 10.1016/j.esmoop.2025.105295. Epub 2025 Jun 3. ESMO Open. 2025. PMID: 40466433 Free PMC article.
-
Anti-PD-1 antibody with or without capecitabine as maintenance therapy after first-line therapy of recurrent or metastatic nasopharyngeal carcinoma.Oncologist. 2025 Jul 4;30(7):oyaf188. doi: 10.1093/oncolo/oyaf188. Oncologist. 2025. PMID: 40577355 Free PMC article.
-
Diagnostic performance of EBV DNA load testing for nasopharyngeal carcinoma in nasopharyngeal swab outperforms the approach in other specimens.BMC Cancer. 2025 Jul 1;25(1):1126. doi: 10.1186/s12885-025-14539-5. BMC Cancer. 2025. PMID: 40597842 Free PMC article.
-
Efficacy and Safety of Immune Checkpoint Inhibitors Combined With Chemotherapy as First-line Treatment for Recurrent or Metastatic Nasopharyngeal Carcinoma: A Network Meta-analysis of Randomized Controlled Trials.Ann Pharmacother. 2024 Apr;58(4):349-359. doi: 10.1177/10600280231188171. Epub 2023 Jul 24. Ann Pharmacother. 2024. PMID: 37488978
References
-
- Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1–Positive, advanced non–Small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. (2020) 38:1580–90. doi: 10.1200/JCO.19.02446 - DOI - PubMed
-
- Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2021) 22:1162–74. doi: 10.1016/S1470-2045(21)00302-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

