Unleashing the power of CAR-M therapy in solid tumors: a comprehensive review
- PMID: 40574837
- PMCID: PMC12198237
- DOI: 10.3389/fimmu.2025.1615760
Unleashing the power of CAR-M therapy in solid tumors: a comprehensive review
Abstract
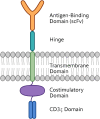
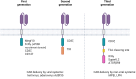
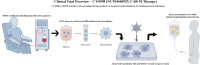
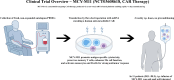
Chimeric antigen receptor (CAR) macrophage therapy represents a promising new frontier in cancer immunotherapy, with the potential to overcome the limitations of CAR-T cell approaches, particularly in solid tumours. This comprehensive review focuses on the current state and future prospects of CAR macrophage technology, emphasising its applications in solid malignancies across preclinical and early clinical development. The key topics covered included CAR design optimisation, macrophage sources and engineering strategies, mechanisms of antitumour activity, in vivo efficacy in animal models, initial clinical trial results, and challenges for broader implementation. The unique properties of macrophages, including tumour penetration and microenvironment modulation, offer significant advantages over T cell-based therapies in solid-tumour settings. However, strategies to enhance persistence, maintain proinflammatory phenotypes, and improve manufacturing are required. Although early research suggests additional applications beyond oncology, including for infectious and inflammatory diseases, this review primarily concentrates on the oncologic potential of CAR-M therapies. Continued optimisation and larger randomised trials will be critical to establish clinical efficacy and define the role of this approach in the treatment of solid tumours.
Keywords: CAR-M; advances in research; cellular immunotherapy; chimeric antigen receptor; macrophages.
Copyright © 2025 Morva, Arroyo, Andreeva, Tapia-Abellán and Luengo-Gil.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

