Cancer cell-derived extracellular vesicles: a potential target for overcoming tumor immunotherapy resistance and immune evasion strategies
- PMID: 40574844
- PMCID: PMC12198139
- DOI: 10.3389/fimmu.2025.1601266
Cancer cell-derived extracellular vesicles: a potential target for overcoming tumor immunotherapy resistance and immune evasion strategies
Abstract
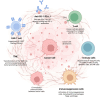
Extracellular vesicles (EVs), including exosomes and microvesicles, play crucial roles in cancer progression by mediating the communication between cancer cells and their microenvironment. Cancer cell-derived EVs promote tumor growth, metastasis, and immune evasion by carrying bioactive materials, such as proteins, RNAs, DNA fragments, and lipids but, immunotherapy aims to enhance the immune response against cancer; however, resistance remains a major challenge. Cancer cell-derived EVs contribute to this resistance by delivering immunosuppressive molecules that impair T cell activation, promote the expansion of regulatory T cells (Tregs), and reduce natural killer (NK) cell cytotoxicity, thereby allowing cancer cells to evade immune surveillance. Additionally, cancer cell-derived EVs can carry immune checkpoint proteins, such as Programmed Death-Ligand 1 (PD-L1), which bind to the Programmed Death-1 (PD-1) receptor on T cells, leading to T cell exhaustion and reduced anti-tumor activity. This mechanism reflects how cancer cells directly evade immune detection and contributes to the overall resistance to immune checkpoint blockade therapies, such as anti-PD-1 or anti-PD-L1 antibodies. By delivering these immunomodulatory molecules, EVs not only contribute to local immune suppression but also create a systemic environment that is less favorable for effective anticancer immunity. Therefore, understanding the role of EVs in the immunotherapy resistance is crucial for developing targeted strategies to counteract their effects and ultimately improve therapeutic outcomes. Here we encourage researchers to pay more attention to the role of cancer cell-derived EVs in overcoming immunotherapeutic resistance, because such efforts may be one of the most promising approaches to address immunotherapy resistance in the future.
Keywords: cancer therapy; extracellular vesicles; immune cells; immune checkpoint inhibitors; immunotherapeutic resistance; tumor microenvironment.
Copyright © 2025 Ahn, Mun, Han and Seo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Co-delivery of axitinib and PD-L1 siRNA for the synergism of vascular normalization and immune checkpoint inhibition to boost anticancer immunity.J Nanobiotechnology. 2025 Mar 10;23(1):194. doi: 10.1186/s12951-025-03170-y. J Nanobiotechnology. 2025. PMID: 40059141 Free PMC article.
-
High matrix metalloproteinase-2 expression predicts poor prognosis of colon adenocarcinoma and is associated with PD-L1 expression and lymphocyte infiltration.PeerJ. 2025 Jun 30;13:e19550. doi: 10.7717/peerj.19550. eCollection 2025. PeerJ. 2025. PMID: 40611940 Free PMC article.
-
Mechanisms, combination therapy, and biomarkers in cancer immunotherapy resistance.Cell Commun Signal. 2024 Jun 19;22(1):338. doi: 10.1186/s12964-024-01711-w. Cell Commun Signal. 2024. PMID: 38898505 Free PMC article. Review.
-
Cytokine-driven cancer immune evasion mechanisms.Int Rev Cell Mol Biol. 2025;396:1-54. doi: 10.1016/bs.ircmb.2025.01.004. Epub 2025 Feb 22. Int Rev Cell Mol Biol. 2025. PMID: 40701694 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

