Immune monitoring and risk of infection in pediatric liver transplantation: a prospective study
- PMID: 40574851
- PMCID: PMC12197944
- DOI: 10.3389/fimmu.2025.1605716
Immune monitoring and risk of infection in pediatric liver transplantation: a prospective study
Abstract
Background: Immune monitoring has been proposed to optimize immunosuppressive therapy in liver recipients. This study aims to describe immunological changes following liver transplantation in pediatric recipients and to identify immune markers associated with post-transplant complications.
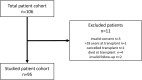
Methods: The immunological status of 95 pediatric liver recipients was prospectively assessed before transplantation and at 1, 3, 6, 9 and 12 months post-transplantation. Serum immunoglobulins (Ig) were measured by nephelometry and immunophenotype was evaluated by flow cytometry. T, B and NK lymphocyte counts were adjusted for age using standard reference ranges.
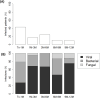
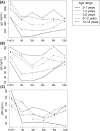
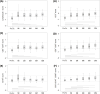
Results: Graft rejection, post-transplant lymphoproliferative disorder and autoimmune hepatitis was diagnosed in 6%, 2% and 0% patients, respectively. Early infections affected 43% patients, while late infections occurred in 17%, 24%, 10% and 9% recipients at each follow-up interval. Baseline immune dysregulation primarily involved the cellular compartment, with 78% recipients showing lymphopenia. Lymphocyte subpopulation scores improved following liver transplantation, with CD4+ score normalizing by month 1 and CD8+, CD19+ and NK scores by month 6. First-month IgG hypogammaglobulinemia, observed in 20% recipients, resolved completely at month 12. First-month T-cell lymphopenia (CD3+ hazard ratio [HR] 2.48, p=0.005; CD8+ HR 2.38, p=0.008) and hypogammaglobulinemia (IgG HR 2.18, p=0.036; IgA HR 2.40, p=0.011; IgM HR 2.61, p=0.006) were associated with higher risk of late infections. In multivariate analysis, only CD3+ T-cell lymphopenia remained a significant predictor (HR 2.13, p=0.030).
Conclusions: Baseline immune dysregulation resolved within the first months post-transplantation. Early infections were unrelated to immune markers, while late infections were associated with CD3+ T-cell lymphopenia and hypogammaglobulinemia.
Keywords: cellular immunity; flow cytometry; humoral immunity; immune monitoring; liver transplantation.
Copyright © 2025 Cuesta-Martín de la Cámara, Miguel-Berenguel, Cámara, Losantos-García, Frauca-Remacha, Hierro-Llanillo, Muñoz-Bartolo, Lledín-Barbacho, Martínez-Feito, López-Granados and Sánchez-Zapardiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Jul 20;7(7):CD004756. doi: 10.1002/14651858.CD004756.pub4. Cochrane Database Syst Rev. 2017. PMID: 28731207 Free PMC article.
-
Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Jan 11;1(1):CD004759. doi: 10.1002/14651858.CD004759.pub2. Cochrane Database Syst Rev. 2017. PMID: 28073178 Free PMC article.
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26;483(9):1680-95. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Persistent lymphopenia after kidney transplantation: increased mortality and decreased homeostatic mechanisms.Front Immunol. 2025 Jun 19;16:1605794. doi: 10.3389/fimmu.2025.1605794. eCollection 2025. Front Immunol. 2025. PMID: 40612959 Free PMC article.
-
Interleukin 2 receptor antagonists for kidney transplant recipients.Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD003897. doi: 10.1002/14651858.CD003897.pub3. Cochrane Database Syst Rev. 2010. PMID: 20091551 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

