Radiation therapy induced intestinal barrier damage and repair process - differences in salivary metabolites and monitoring of intestinal barrier function
- PMID: 40574858
- PMCID: PMC12197936
- DOI: 10.3389/fimmu.2025.1590219
Radiation therapy induced intestinal barrier damage and repair process - differences in salivary metabolites and monitoring of intestinal barrier function
Abstract
Purpose: Colorectal cancer (CRC) is still one of the most common malignant tumors, with gradual increase in its annual morbidity and mortality. But most cases are diagnosed in the late stage. For stage II-III cancer, clinical guidelines recommend surgery following neoadjuvant radiation therapy at ≥6 weeks after the last radiotherapy is completed. However, radiotherapy may impair intestinal mucosal barrier function, especially the biological and immune barriers, accompanied by perioperative complications. This study was conducted to investigate the changes, repair patterns, and potential mechanisms in patients after radiotherapy.
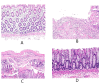
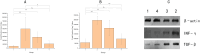
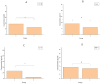
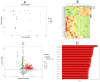
Methods: This study detected inflammatory factors in postoperative intestinal mucosal tissue and serum, as well as metabolites in saliva samples, and collected hematoxylin-eosin (HE)-stained pathological images in CRC patients who had received and did not receive radiotherapy.
Results: The results showed that after radiotherapy, there were significantly impaired intestinal mucosal tissue structure; obviously elevated inflammatory factors in intestinal mucosal tissue and blood; as well as upregulation/downregulation of metabolites in saliva samples.
Conclusion: In conclusion, findings in this study may provide potential reference for predicting the recovery of intestinal mucosa and selecting the optimal timing for surgery after radiotherapy. In addition, this study will benefit the understanding and reduction of perioperative complications caused by intestinal barrier damage.
Keywords: biological barrier; damage and repair; immune barrier; intestinal mucosal barrier; radiotherapy; rectal cancer; salivary metabolites.
Copyright © 2025 Jingjing, Kun, Yanyu, Mengjie, Yunqing, Yulong, Xuelong, Xiaojie, Haitao and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy.Cochrane Database Syst Rev. 2017 Jul 31;7(7):CD012744. doi: 10.1002/14651858.CD012744. Cochrane Database Syst Rev. 2017. PMID: 28759701 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

