The potential dual role of tau phosphorylation: plasma phosphorylated-tau217 in newborns and Alzheimer's disease
- PMID: 40574977
- PMCID: PMC12198956
- DOI: 10.1093/braincomms/fcaf221
The potential dual role of tau phosphorylation: plasma phosphorylated-tau217 in newborns and Alzheimer's disease
Abstract
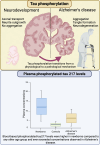
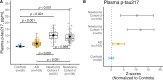
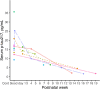
Tau phosphorylation plays an important role in brain physiology and pathology. During foetal development, it supports microtubule dynamics and neuroplasticity, whereas in Alzheimer's disease (AD), it drives pathological tau aggregation and tangle formation. In this multicentre study (n = 462), we measured plasma phosphorylated-tau217 in healthy newborns, premature infants, patients with AD and healthy controls across various age groups. Plasma phosphorylated-tau217 levels were significantly higher in newborns compared to healthy individuals of any age group and even exceeded levels observed in patients with AD. In newborns, plasma phosphorylated-tau217 levels inversely correlated with perinatal factors such as gestational age. Longitudinal analysis of preterm infants demonstrated a decline in serum phosphorylated-tau217 levels over the first months of life, approaching levels observed in young adults. In contrast, elevated plasma phosphorylated-tau217 in older individuals was associated with AD pathology. Our findings corroborate the crucial role of tau phosphorylation in early brain development. However, in AD, tau phosphorylation transitions into a pathological mechanism. The high levels of blood-based phosphorylated-tau217 observed at birth and subsequent clearance might indicate distinct regulatory mechanisms that prevent tau aggregation in early life. Further studies are needed to explore the shared mechanisms of tau phosphorylation in newborns and AD.
Keywords: Alzheimer’s disease; newborns; phosphorylated tau; plasma biomarkers.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.T. and P.H. are employees of Bioventix. HZ has served at scientific advisory boards and/or as a consultant for AbbVie Inc, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Enigma, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche and WebMD and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and at advisory boards for AbbVie, AC Immune, ALZpath, AriBio, Beckman-Coulter, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Quanterix, Roche Diagnostics, Sanofi and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programmes for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. M.S.-C. has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and Grifols S.L., has given lectures in symposia sponsored by Roche Diagnostics and Roche Farma, S.A. and was granted with a project funded by Roche Diagnostics International Ltd; payments were made to the institution (Barcelona Beta Research Center). B.-E.K. has served as a consultant for Biogen and advisory board for Eisai. T.F. has served as a consultant and at the advisory boards for Biogen, Novo Nordisk, Eli Lilly and Roche. The other authors declare no competing interest.
Figures

References
-
- Parra Bravo C, Naguib SA, Gan L. Cellular and pathological functions of tau. Nat Rev Mol Cell Biol. 2024;25(11):845–864. - PubMed
-
- Brion JP, Smith C, Couck AM, Gallo JM, Anderton BH. Developmental changes in τ phosphorylation: Fetal τ is transiently phosphorylated in a manner similar to paired helical filament-τ characteristic of Alzheimer’s disease. J Neurochem. 1993;61(6):2071–2080. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103(1):26–35. - PubMed
LinkOut - more resources
Full Text Sources
