Regulation of bacterial phosphorelay systems
- PMID: 40575134
- PMCID: PMC12189002
- DOI: 10.1039/d5cb00016e
Regulation of bacterial phosphorelay systems
Abstract
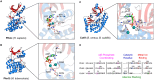
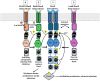
In terms of biomass, bacteria are the most successful organisms on earth. This is partly attributed to their tremendous adaptive capabilities, which allows them to sense and rapidly organise responses to changing environmental stimuli. Using complex signalling mechanisms, bacteria can relay cellular information to fine-tune their metabolism, maintain homeostasis, and trigger virulence processes during infection. Across all life, protein phosphorylation represents the most abundant signalling mechanism, which is controlled by a versatile class of enzymes called protein kinases and their cognate phosphatases. For many years, histidine kinase (HK)-containing two-component systems (TCSs) were considered the canonical instruments of bacterial sensing. However, advances in metagenomics has since proven that bacterial phosphorelay is in fact orchestrated by a functionally diverse array of integrated protein kinase types, including Ser, Thr, Tyr and Arg-targeting enzymes. In this review, we provide an up-to-date appraisal of bacterial kinase signalling, with an emphasis on how these sensing pathways are regulated to modulate kinase output. Finally, we explore how selective kinase inhibitors may be exploited to control infections and combat the looming health emergency of multidrug resistant bacteria.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Krebs E. G. Fischer E. H. The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochem. Biophys. Acta. 1956;20:150–157. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

