Lung cancer-associated fibroblasts-mediated collagen deposition drives mediastinal lymph node metastasis in non-small cell lung cancer
- PMID: 40575163
- PMCID: PMC12198238
- DOI: 10.3389/fonc.2025.1597585
Lung cancer-associated fibroblasts-mediated collagen deposition drives mediastinal lymph node metastasis in non-small cell lung cancer
Abstract
Introduction: Metastasis to mediastinal lymph nodes signifies an advanced stage of non-small cell lung cancer (NSCLC) and presents significant clinical challenges. Cancer-associated fibroblasts (CAFs) within the tumor microenvironment (TME) play a crucial role in tumor progression by promoting growth and invasion. However, the specific contributions of lung CAFs to mediastinal lymph node metastasis in NSCLC remain poorly understood. Moreover, no therapeutics currently target CAFs to combat mediastinal lymph node metastasis in NSCLC. This study aims to elucidate the precise roles of CAFs in these complex processes and to investigate innovative therapeutic strategies that target CAFs to suppress metastasis to mediastinal lymph nodes.
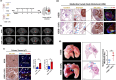
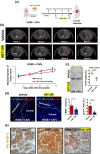
Methods: Normal human lung fibroblasts (MRC-5) were directly co-cultured with NSCLC cell lines (H358 and HCC827) to generate lung CAFs. These activated CAFs were identified using immunofluorescence, flow cytometry, and Western blotting. To model human mediastinal lymph node metastasis, orthotopic xenograft (OX) models were established by intrathoracically injecting NSCLC cells into the left lung of mice, either alone or in combination with lung CAFs. The viability of cancer cells and lung CAFs post-treatment with ABT-199, a Bcl-2 inhibitor, was evaluated using MTT assays. The therapeutic efficacy of ABT-199 was further assessed through oral gavage in OX models, focusing on its potential to prevent metastasis to mediastinal lymph nodes. Tumor growth was monitored longitudinally using micro-computed tomography (CT), and treatment response was evaluated in accordance with RECIST 1.1 criteria. Primary tumors and mediastinal lymph node metastases were analyzed using hematoxylin and eosin (H&E) staining for general morphology, supplemented by immunohistochemistry/immunofluorescence to detect specific protein markers. Fibrillar collagen deposition within the TME was quantified using picrosirius red staining (PRS).
Results: Activation of α-SMA, accompanied by a significant increase in Col 1A1 expression in MRC-5 cells, was successfully induced through a direct 14-day co-culture with NSCLC cell lines. Histological analysis revealed increased fibrotic tissue formation, enhanced fibrillar collagen deposition, peritumoral lymphangiogenesis, and mediastinal lymph node metastasis in CAFs-enriched orthotopic xenograft (OX) tumors compared to CAFs-devoid OX tumors. The viability of lung CAFs was dose-dependently inhibited by ABT-199 in vitro. A two-week treatment with ABT-199 (100mg/kg) led to a significant reduction in lung CAFs and lymphangiogenesis in the CAFs-enriched OX model. Furthermore, an eight-week treatment with ABT-199 (100mg/kg) significantly reduced fibrillar collagen deposition and inhibited the number of metastases to mediastinal lymph nodes in both CAFs-devoid and CAFs-enriched OX models.
Conclusions: In NSCLC, cancer cells induce the differentiation of resident normal lung fibroblasts into lung CAFs. These CAFs secrete excessive collagens within the TME, thereby promoting tumor lymphangiogenesis and facilitating metastasis to mediastinal lymph nodes. Our findings, based on modified OX models, suggest that targeting lung CAFs could effectively attenuate lymphatic dissemination in NSCLC.
Keywords: NSCLC; cancer-associated fibroblasts; lymphangiogenesis; mediastinal lymph node metastasis; orthotopic xenograft model.
Copyright © 2025 Chen, Feng, Ko, Lam, Yan and Ho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Integrin αVβ1-activated PYK2 promotes the progression of non-small-cell lung cancer via the STAT3-VGF axis.Cell Commun Signal. 2024 Jun 6;22(1):313. doi: 10.1186/s12964-024-01639-1. Cell Commun Signal. 2024. PMID: 38844957 Free PMC article.
-
Investigation of sentinel lymph nodes using SPECT/CT and perioperative gamma assay combined with immunohistochemistry in non-small cell lung cancer patients undergoing lung resection.Indian J Thorac Cardiovasc Surg. 2025 Jul;41(7):874-881. doi: 10.1007/s12055-025-01924-9. Epub 2025 Mar 22. Indian J Thorac Cardiovasc Surg. 2025. PMID: 40535215
-
Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2011 Jan;15(4):iii-iv, 1-134. doi: 10.3310/hta15040. Health Technol Assess. 2011. PMID: 21276372 Free PMC article.
-
EMT induction in normal breast epithelial cells by COX2-expressing fibroblasts.Cell Commun Signal. 2025 May 22;23(1):237. doi: 10.1186/s12964-025-02227-7. Cell Commun Signal. 2025. PMID: 40405218 Free PMC article.
-
Markers of breast cancer stromal fibroblasts in the primary tumour site associated with lymph node metastasis: a systematic review including our case series.Biosci Rep. 2013 Dec 12;33(6):e00085. doi: 10.1042/BSR20130060. Biosci Rep. 2013. PMID: 24229053 Free PMC article.
References
-
- Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metast Rev. (1989) 8:98–101. - PubMed
LinkOut - more resources
Full Text Sources

