Intratumoral microbiota composition in women's cancers: a systematic review and meta-analysis
- PMID: 40575164
- PMCID: PMC12197917
- DOI: 10.3389/fonc.2025.1544786
Intratumoral microbiota composition in women's cancers: a systematic review and meta-analysis
Abstract
Background: The intratumoral microbiota has attracted considerable interest in carcinogenesis, progression, and treatment owing to advancements in sequencing technology. This systematic review provides a comprehensive overview of the current literature regarding the diversity and compositional characteristics of the intratumoral microbiota in women's cancers. Additionally, it also explores potential associations among intratumoral microbiota, estrogen, and anti-tumor therapies.
Methods: A comprehensive literature search was conducted using PubMed, Embase, Web of Science, and the Cochrane Library from their inception to May 1, 2024. The review protocol was pre-registered in PROSPERO (CRD 42024601213). Articles were assessed utilizing the Newcastle-Ottawa Scale (NOS). To estimate the effect size and variability in microbial diversity changes, the standardized mean difference (SMD) and 95% confidence intervals (CIs) were employed. The systematic review adhered to PRISMA reporting guidelines, and meta-analyses were performed using Review Manager version 5.4.
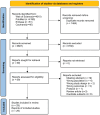
Results: This systematic review included 29 of 8,291 studies after a thorough screening process. Of the 22 studies investigating α-diversity in women's cancers, disease-free controls, and those with benign conditions, notable changes in diversity indices were observed. Compared to adjacent normal tissues, the Simpson index significantly decreased in breast cancer (SMD = -0.75, 95% CI: [-0.94, -0.55]) and endometrial cancer (SMD = -0.83, 95% CI: [-1.37, -0.28]). The Chao1 index was reduced in endometrial cancer tumor tissues relative to normal tissues (SMD = -2.25, 95% CI: [-3.13, -1.36]), while the Shannon index decreased in ovarian cancer tumor tissues (SMD = -0.61, 95% CI: [-1.18, -0.04]). In comparisons between tumor and benign tissues, the Chao1 index was decreased (SMD = -0.64, 95% CI: [-1.20, -0.08], I² = 0%), while the Simpson index was increased (SMD = 0.36, 95% CI: [0.01, 0.71], I² = 0%) in patients with ovarian cancer. Other microbial diversity indices showed no significant differences between tumor and non-tumor tissues. At the phylum level, Fusobacteriota were enriched in tumor tissues, while Firmicutes and Actinobacteria predominated in non-tumor tissues. At the genus level, Pseudomonas, Porphyromonas, Atopobium, Peptoniphilus, and Acinetobacter were consistently more abundant in cancerous tissues. Microbial alterations were also linked to estrogen receptor (ER) status, with Alkanindiges negatively correlated with ER status in two studies. Furthermore, one study on the effect of antineoplastic therapy indicated that neoadjuvant chemotherapy reduced microbial diversity in breast cancer patients (n = 15 vs. n = 18) (Shannon index: SMD = -0.95, 95% CI: [-1.68, -0.22]).
Conclusion: This study highlights significant differences in microbiota composition between tumor and non-tumor tissues in women's cancers, emphasizing changes in intratumoral microbiota influenced by estrogen and antineoplastic treatments. Further research is needed to explore the potential for developing targeted therapies based on estrogen-driven microbiota alterations. Investigations may yield insights into the enhancement of female reproductive health and the improvement of treatment efficacy for female cancers.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024601213, identifier CRD 42024601213.
Keywords: 16s rrna gene sequencing; breast cancer; estrogen; gynecologic cancer; intratumoral microbiota; meta-analysis.
Copyright © 2025 Wen, Wang, Fu, Zhou, Min, Lang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Selective progesterone receptor modulators (SPRMs) for uterine fibroids.Cochrane Database Syst Rev. 2017 Apr 26;4(4):CD010770. doi: 10.1002/14651858.CD010770.pub2. Cochrane Database Syst Rev. 2017. PMID: 28444736 Free PMC article.
-
Exercise interventions on health-related quality of life for people with cancer during active treatment.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. Cochrane Database Syst Rev. 2012. PMID: 22895974 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

