A Rare Case of Methyldopa-Induced Hepatitis
- PMID: 40575235
- PMCID: PMC12199078
- DOI: 10.7759/cureus.84873
A Rare Case of Methyldopa-Induced Hepatitis
Abstract
Methyldopa is a centrally acting antihypertensive agent commonly used in the management of hypertension. It has been associated with rare but serious hepatotoxic effects. Methyldopa-induced hepatitis is an uncommon but potentially devastating adverse effect that can mimic autoimmune hepatitis (AIH) both clinically and histologically. This report presents the case of a 31-year-old woman with a history of hypertension who developed acute hepatitis following the initiation of methyldopa. The patient presented with fatigue, jaundice, and elevated liver enzymes approximately six weeks after starting therapy. Viral, autoimmune, and metabolic causes of hepatitis were excluded through comprehensive testing. Liver function normalized following the discontinuation of methyldopa, and the patient recovered fully, supporting a diagnosis of drug-induced liver injury (DILI). Causality was assessed using the Roussel Uclaf Causality Assessment Method (RUCAM), yielding a score of 9, indicating a 'highly probable' link between methyldopa and the observed hepatitis.
Keywords: dili; hepatitis; jaundice; liver failure; methyldopa.
Copyright © 2025, Bonito et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
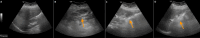
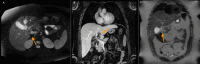
Similar articles
-
Liver Injury in COVID-19 Patients with Drugs as Causatives: A Systematic Review of 996 DILI Cases Published 2020/2021 Based on RUCAM as Causality Assessment Method.Int J Mol Sci. 2022 Apr 27;23(9):4828. doi: 10.3390/ijms23094828. Int J Mol Sci. 2022. PMID: 35563242 Free PMC article.
-
Methyldopa for primary hypertension.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD003893. doi: 10.1002/14651858.CD003893.pub3. Cochrane Database Syst Rev. 2009. PMID: 19821316 Free PMC article.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Glucocorticosteroids for people with alcoholic hepatitis.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD001511. doi: 10.1002/14651858.CD001511.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Apr 09;4:CD001511. doi: 10.1002/14651858.CD001511.pub4. PMID: 29096421 Free PMC article. Updated.
References
-
- Methyldopa-induced acute toxic hepatitis [Article in Spanish] Fernández-Marcote Menor EM, Pérez-Bedmar Delgado J. Rev Esp Enferm Dig. 2005;97:840–841. - PubMed
-
- Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Clin Gastroenterol Hepatol. 2017;15:1635–1636. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
