A Cost-Effectiveness Analysis of Diffuse Large B-Cell Lymphoma Treatment Pathways in the United States
- PMID: 40575250
- PMCID: PMC12198509
- DOI: 10.1177/23814683251345780
A Cost-Effectiveness Analysis of Diffuse Large B-Cell Lymphoma Treatment Pathways in the United States
Abstract
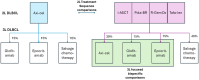
Background. Chimeric antigen receptor (CAR) T-cell therapies are approved as second-line (2L) or later therapy for diffuse large B-cell lymphoma (DLBCL). Recently, bispecific T-cell antibodies (BsAbs) have been approved as third-line (3L) treatments. The cost-effectiveness of different treatment sequences is unknown. This study aims to evaluate the cost-effectiveness of axicabtagene ciloleucel (axi-cel) compared with other treatment options for 2L DLBCL, from a US health care perspective at a cost-effectiveness threshold of $150,000 per quality-adjusted life-year (QALY). Design. This economic evaluation used a discrete event simulation decision. Model inputs were derived from 8 clinical trials and the published literature. Simulated patients received 2L axi-cel followed by 3L treatments, which were compared with treatment sequences of 2L intended autologous stem cell transplant (ASCT), polatuzumab vedotin with bendamustine and rituximab (Pola-BR), tafasitamab with lenalidomide (tafa-len), or rituximab with gemcitabine and oxaliplatin (R-GemOx), all of which were followed by 3L treatments (salvage chemotherapy, BsAbs, or axi-cel). In addition, axi-cel was compared directly with glofitamab and epcoritamab in 3L. Costs and QALYs, discounted at 3.0%, were used to derive incremental cost-effectiveness ratios (ICERs) and net monetary benefits (NMBs). Results. In the 2L base case, axi-cel was cost-effective compared with intended ASCT (ICER $145,004/QALY), which was cost-effective compared with R-GemOx (ICER $9,495/QALY). Axi-cel maximized NMB at $150,000 and $200,000/QALY thresholds, whereas intended ASCT maximized NMB at $100,000/QALY. In 3L-focused comparisons with epcoritamab and glofitamab, axi-cel was dominant and cost-effective (ICER $122,224/QALY), respectively. Axi-cel maximized NMB at $150,000 and $200,000/QALY thresholds, whereas glofitamab maximized NMB at $100,000/QALY. Conclusions. The findings of the study suggest that although other treatments were cost-effective at lower thresholds, axi-cel is a cost-effective treatment option in 2L/3L settings in the United States.
Highlights: This study investigated whether axicabtagene ciloleucel (axi-cel) is cost-effective in second-line (2L) and third-line (3L) treatment sequences in the current relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) treatment paradigm.Using a novel treatment sequencing model, axi-cel was found to be cost-effective in both 2L treatment sequences and in direct comparisons with 3L bispecific T-cell antibodies.These findings suggest that axi-cel is a cost-effective treatment for R/R DLBCL regardless of treatment line positioning.
Keywords: CAR T-cell therapy; axicabtagene ciloleucel; bispecific antibody therapy; cost-effectiveness; epcoritamab; glofitamab; lymphoma; mixture cure model; survival analysis.
© The Author(s) 2025.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kievit, Sharma, Hofmann (former), and Blissett are employees of Maple Health Group, who were contracted by Kite Pharma to conduct the work contained in this article. Patel (former), Hasegawa, and Ray are employees of Kite Pharma. Locke received consulting fees from Kite, A Gilead Company; A2; Allogene; Amgen; Bluebird Bio; Bristol-Myers Squibb; Calibr; Caribou; Cowen; EcoR1; Gerson Lehrman Group; Iovance; Janssen; Legend Biotech; Novartis; Sana; Umoja; and Pfizer. Locke received institutional support from the Society for Immunotherapy of Cancer, Leukemia and Lymphoma Society, and National Cancer Institute. Locke has patents under his name. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided entirely by a contract with Kite, A Gilead Company. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Patel, Hasegawa, and Ray.
Figures

Similar articles
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Health and Economic Impact of Vein-to-Vein Time in CAR T-Cell Therapy in the Second-Line Treatment of Relapsed/Refractory Large B-Cell Lymphoma: A US Cost-Effectiveness Analysis.Transplant Cell Ther. 2025 May 13:S2666-6367(25)01166-2. doi: 10.1016/j.jtct.2025.05.002. Online ahead of print. Transplant Cell Ther. 2025. PMID: 40373976
-
Clinical representativeness of pivotal trials for T-cell engagers in relapsed/refractory follicular lymphoma.Future Oncol. 2025 Aug 13:1-7. doi: 10.1080/14796694.2025.2543673. Online ahead of print. Future Oncol. 2025. PMID: 40799045
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
LinkOut - more resources
Full Text Sources

