A Remarkable Catalyst-Free Photochemical Alkene Hydrophosphination with Bis(trimethylsilyl)phosphonite
- PMID: 40575286
- PMCID: PMC12188422
- DOI: 10.1021/jacsau.5c00444
A Remarkable Catalyst-Free Photochemical Alkene Hydrophosphination with Bis(trimethylsilyl)phosphonite
Abstract
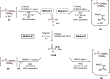
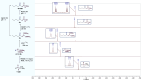
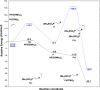
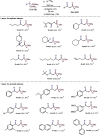
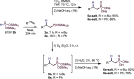
This publication delves into a comprehensive exploration of a new synthetic route to functionalized phosphorus-derived compounds. Bis-(trimethylsilyl)-phosphonite HP-(OSiMe3)2, prepared in a high yield from H3PO2, was found to be an excellent reagent for hydrophosphination of activated, unactivated, and amino acid-derived olefins under UV irradiation and catalyst-, solvent- and glovebox-free conditions. The resulting phosphorus trivalent compounds have been subjected to postfunctionalization, leading to the formation of H-phosphinate, phosphonate, and thiophosphonate end products in good to excellent yields. With a combination of experimental and theoretical calculations, the mechanism for this hydrophosphination reaction has been investigated, revealing a radical process.
Keywords: P-chemicals; bis(trimethylsilyl)phosphonite; catalyst-free; divergent synthesis; hydrophosphination; photochemistry.
© 2025 The Authors. Published by American Chemical Society.
Figures


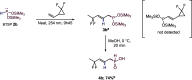


References
-
- Demkowicz S., Rachon J., Daśko M., Kozak W.. Selected Organophosphorus Compounds with Biological Activity. Applications in medicine. RSC Adv. 2016;6:7101. doi: 10.1039/C5RA25446A. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
