In Vivo Visualization of Tumor Metabolic Activity with a Tetra Glucose-Conjugated Zinc-Phthalocyanine Photoacoustic Contrast
- PMID: 40575291
- PMCID: PMC12188402
- DOI: 10.1021/jacsau.5c00151
In Vivo Visualization of Tumor Metabolic Activity with a Tetra Glucose-Conjugated Zinc-Phthalocyanine Photoacoustic Contrast
Abstract
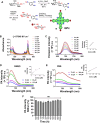
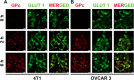
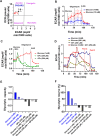
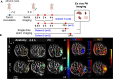
Tissue metabolic alterations are associated with tumor progression and serve as clinical biomarkers. Molecular imaging methods can provide a noninvasive assessment of this altered metabolic activity. Currently, in the clinic, nuclear medicine using 18F-FDG, a radioactive analogue of glucose, is the gold standard for visualizing metabolically active tumors. However, the accompanying ionizing radiation and accumulated radiation dosage limit its unchartered use. Noninvasive imaging of tissue metabolic activity without incorporating any radioactive isotope or another additional anatomical imaging is a promising alternative to nuclear medicine. Here, we introduce the first-of-its-kind tetra glucose-conjugated molecular photoacoustic (PA) contrast agent, a water-soluble and biocompatible small molecule based on the Zn-phthalocyanine scaffold. Although the Zn-phthalocyanine core is hydrophobic, the conjugation of four glucose units through their anomeric carbon ensured the water solubility of this agent, thereby aiding in its potential translation for in vivo studies. In addition, such a conjugation contributed to the high cellular uptake of this molecule in two aerobic cancer cell lines, as demonstrated using flow cytometry and epifluorescence microscopy studies. Importantly, with live metabolic assays, we elucidated the mechanism through which the contrast agent could be utilized as a glucose antagonist in nutrient-starved cells. Finally, with real-time in vivo PA tomography studies in a 4T1 mouse tumor model, we showed maximum agent accumulation within 4 h and tumor washout within 12 h post intravenous administration. Noninvasive molecular PA imaging of metabolic tumors with this probe offers a promising alternative to nuclear medicine, especially in assessing therapy response with the requirement of shorter intervals for follow-up in the clinic.
Keywords: in vivo metabolic imaging; photoacoustic imaging; phthalocyanines; small-molecule contrast; tumor metabolism.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Methods for blood loss estimation after vaginal birth.Cochrane Database Syst Rev. 2018 Sep 13;9(9):CD010980. doi: 10.1002/14651858.CD010980.pub2. Cochrane Database Syst Rev. 2018. PMID: 30211952 Free PMC article.
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2022 Sep 2;9(9):CD013483. doi: 10.1002/14651858.CD013483.pub2. Cochrane Database Syst Rev. 2022. PMID: 36053210 Free PMC article.
References
-
- Faubert B., DeBerardinis R. J.. Analyzing Tumor Metabolism In Vivo. Annu. Rev. Cancer Biol. 2017;1:99–117. doi: 10.1146/annurev-cancerbio-050216-121954. - DOI
LinkOut - more resources
Full Text Sources
