Leveraging the Aminothiol-Specific Phosphorogenic Response of Iridium(III) Thioester Complexes for the Development of Intracellular Sensors and Cancer Phototherapeutics
- PMID: 40575299
- PMCID: PMC12188478
- DOI: 10.1021/jacsau.5c00413
Leveraging the Aminothiol-Specific Phosphorogenic Response of Iridium(III) Thioester Complexes for the Development of Intracellular Sensors and Cancer Phototherapeutics
Abstract
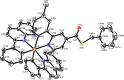
Site-specific bioconjugation techniques are extensively utilized in biological and biomedical fields to precisely label biomolecules with luminescent tags for direct visualization of their intracellular dynamics or with cytotoxic agents for the development of novel anticancer therapeutics. In this work, a series of cyclometalated iridium-(III) polypyridine complexes featuring a thioester moiety was designed as novel phosphorogenic probes for labeling N-terminal cysteine (N-Cys)-containing biomolecules. These thioester complexes were weakly emissive in solutions due to the presence of a low-lying nonradiative distorted triplet intraligand (3IL) state localized on the thioester unit, as elucidated by computational analyses. However, their emission intensities and singlet oxygen (1O2)-photosensitization efficiencies substantially increased upon reaction with l-Cys due to the conversion of the quenching thioester moiety to a nonquenching amide unit. Additionally, the thioester complexes exhibited high selectivity toward N-Cys and displayed significantly enhanced reactivity due to the electron-withdrawing iridium-(III) polypyridine moiety. The remarkable aminothiol-induced emission and 1O2-photosensitization turn-on of the thioester complexes were exploited for the development of intracellular Cys sensors and Cys-activatable photosensitizers for cancer-targeted photodynamic therapy. Furthermore, one of the thioester complexes was selected to react with various N-Cys-modified tumor-targeting peptides, yielding photofunctional iridium-(III)-peptide conjugates with high 1O2 generation efficiencies. These conjugates retained the tumor-targeting capabilities of the original peptides and showed high specificity for MDA-MB-231 cells compared to MCF-7 and HEK-293 cells, resulting in selective photocytotoxicity toward this triple-negative breast cancer cell line. We believe that our design approach will inspire the development of novel luminogenic thioester-based reagents for bioconjugation, bioimaging, and therapeutic applications.
Keywords: N-terminal cysteine; bioconjugation; bioimaging; intracellular sensing; iridium; phosphorogenic; photosensitizers; thioester.
© 2025 The Authors. Published by American Chemical Society.
Figures
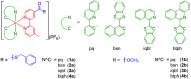







References
-
- Hoyt E. A., Cal P. M. S. D., Oliveira B. L., Bernardes G. J. L.. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 2019;3:147–171. doi: 10.1038/s41570-019-0079-1. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
