Molecularly Defined Glycocalyx Models Reveal AB5 Toxins Recognize Their Target Glycans Superselectively
- PMID: 40575306
- PMCID: PMC12188407
- DOI: 10.1021/jacsau.5c00305
Molecularly Defined Glycocalyx Models Reveal AB5 Toxins Recognize Their Target Glycans Superselectively
Abstract
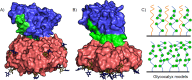
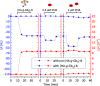
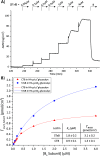
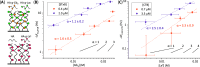
AB5 toxins are a class of bacterial toxins that recognize cell surface carbohydrates to facilitate their uptake by the target cell. Among them are cholera toxin (CT) from Vibrio cholerae that causes cholera, and Shiga toxin (STx) from Shigella dysenteriae and certain strains of Escherichia coli, which cause hemolytic uremic syndrome. While the glycolipid ligands for CT and STx (gangliosides GM1 and Gb3, respectively) have long been known, recent studies have shown that fucosylated structures, like Lewis x (Le x ), also play a role in CT binding. This realization raises questions about the importance of interactions between these toxins and nonglycolipid components of the glycocalyx, which are not well understood. To address this challenge, we created glycocalyx models of defined thickness and tunable molecular composition through grafting of mucin-like glycopolymers on solid-supported lipid bilayers (SLBs). The synthesized mucin-like glycopolymers comprised a hyaluronic acid (HA) backbone, an anchor tag (biotin or hexa-histidine) at the HA reducing end, and side chains of relevant oligosaccharides (Le x , Gb3, or lactose) at defined densities. Analyses by quartz crystal microbalance with dissipation monitoring and spectroscopic ellipsometry provided quantification of the thickness, mesh size, and target glycan concentration of the glycocalyx models and of toxin binding kinetics. The B subunit pentamers of both CT and STx showed significantly enhanced affinity in the model glycocalyx environment due to multivalent binding to their respective target glycans. Most notably, toxin binding increased superlinearly with the concentration of the target glycan in the model glycocalyx. We propose that such "superselective" binding is an important factor in host cell selection. Our approach provides a new set of tools to make designer glycocalyces and analyze multivalent protein-glycan interactions in a controlled environment.
Keywords: QCM-D; biomimetic interfaces; glycoconjugate; lectin binding; superselectivity; synthetic glycocalyx.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article.
References
LinkOut - more resources
Full Text Sources
