Molecularly Defined Glycocalyx Models Reveal AB5 Toxins Recognize Their Target Glycans Superselectively
- PMID: 40575306
- PMCID: PMC12188407
- DOI: 10.1021/jacsau.5c00305
Molecularly Defined Glycocalyx Models Reveal AB5 Toxins Recognize Their Target Glycans Superselectively
Abstract
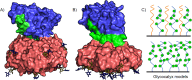
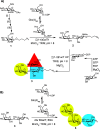
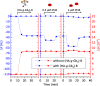
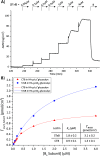
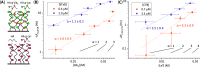
AB5 toxins are a class of bacterial toxins that recognize cell surface carbohydrates to facilitate their uptake by the target cell. Among them are cholera toxin (CT) from Vibrio cholerae that causes cholera, and Shiga toxin (STx) from Shigella dysenteriae and certain strains of Escherichia coli, which cause hemolytic uremic syndrome. While the glycolipid ligands for CT and STx (gangliosides GM1 and Gb3, respectively) have long been known, recent studies have shown that fucosylated structures, like Lewis x (Le x ), also play a role in CT binding. This realization raises questions about the importance of interactions between these toxins and nonglycolipid components of the glycocalyx, which are not well understood. To address this challenge, we created glycocalyx models of defined thickness and tunable molecular composition through grafting of mucin-like glycopolymers on solid-supported lipid bilayers (SLBs). The synthesized mucin-like glycopolymers comprised a hyaluronic acid (HA) backbone, an anchor tag (biotin or hexa-histidine) at the HA reducing end, and side chains of relevant oligosaccharides (Le x , Gb3, or lactose) at defined densities. Analyses by quartz crystal microbalance with dissipation monitoring and spectroscopic ellipsometry provided quantification of the thickness, mesh size, and target glycan concentration of the glycocalyx models and of toxin binding kinetics. The B subunit pentamers of both CT and STx showed significantly enhanced affinity in the model glycocalyx environment due to multivalent binding to their respective target glycans. Most notably, toxin binding increased superlinearly with the concentration of the target glycan in the model glycocalyx. We propose that such "superselective" binding is an important factor in host cell selection. Our approach provides a new set of tools to make designer glycocalyces and analyze multivalent protein-glycan interactions in a controlled environment.
Keywords: QCM-D; biomimetic interfaces; glycoconjugate; lectin binding; superselectivity; synthetic glycocalyx.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
LinkOut - more resources
Full Text Sources
