Dual-Enzyme-Mimicking Sites in Covalent Organic Frameworks Enable Highly Efficient Relay Electrosynthesis of Ammonia
- PMID: 40575315
- PMCID: PMC12188482
- DOI: 10.1021/jacsau.5c00136
Dual-Enzyme-Mimicking Sites in Covalent Organic Frameworks Enable Highly Efficient Relay Electrosynthesis of Ammonia
Abstract
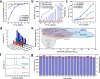
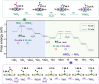
Electrocatalytic nitrate reduction poses significant importance in green ammonia synthesis and water denitrification. To date, high-performance nitrate reduction still relies on noble-metal-based catalysts, while transition metal compound and framework catalysts generally suffer from low activities (in terms of turnover frequency values), leading to insufficient ability to rapidly process large amounts of nitrate at the industrial level. To this end, enzyme-mimicking catalytic systems that integrate the high TOF efficiency of enzymatic sites (typically with nonprecious metal centers) into the crystalline, orderly assembled, and porous molecular frameworks hold great theoretical promises, yet the search for any single enzyme-site mimics has failed to overcome current limitations. Herein, we demonstrate the rational design of a series of covalent organic frameworks (COFs) to incorporate periodically alternating metalloporphyrin and metal-bis-(dithiolene), respectively mimicking nitrate reductase and nitrite reductase enzymes, as a dual functional assembly. The dual-enzyme-mimicking Ni-TAPP-Cu boosts the electrocatalytic activity of NO3RR to a comparable level to the noble-metal catalysts via a relay pathway to individually boost the two kinetically significant nitrate-to-nitrite and nitrite-to-ammonia conversion steps, consequently achieving a remarkable TOF value of 235.7 mg·h-1·mg-1 site, 86.13% NH3 faradaic efficiency, nearly 100% NH3-selectivity, and excellent durability at typical acidic wastewater conditions (pH = 3). Mechanistic investigations indicate that Cu sites effectively suppress the HER competition, enhance the adsorption of NO3 -, and selectively generate NO2 -, while Ni sites facilitate the subsequent hydrogenation and conversion of *NO2. The Cu-Ni-COF exemplifies a promising strategy for integrating dual enzyme-mimicking sites within an ordered, porous framework to facilitate relay catalysis, thereby offering valuable insights for the design of high-performance, sustainable catalytic materials.
Keywords: ammonia electrosynthesis; covalent organic frameworks; dual-enzyme-mimicking; nitrate reduction; non-noble-metal.
© 2025 The Authors. Published by American Chemical Society.
Figures



Similar articles
-
A copper-Zeolitic Imidazolate framework@cobalt-Bipyridine covalent organic framework Heterostructure for efficient tandem catalysis of nitrate Electroreduction to Ammonia.J Colloid Interface Sci. 2025 Dec;699(Pt 2):138258. doi: 10.1016/j.jcis.2025.138258. Epub 2025 Jun 22. J Colloid Interface Sci. 2025. PMID: 40570602
-
Microenvironment Engineering of Mesoporous Metals for Ammonia Electrosynthesis from Nitrate: Advances, Mechanisms, and Prospects.Acc Chem Res. 2025 Jul 15;58(14):2306-2316. doi: 10.1021/acs.accounts.5c00327. Epub 2025 Jul 4. Acc Chem Res. 2025. PMID: 40613651
-
Cascade Electrocatalytic Reduction of Nitrate to Ammonia Using Bimetallic Covalent Organic Frameworks with Tandem Active Sites.Angew Chem Int Ed Engl. 2025 Aug 4;64(32):e202507956. doi: 10.1002/anie.202507956. Epub 2025 Jun 18. Angew Chem Int Ed Engl. 2025. PMID: 40471854 Free PMC article.
-
Conversion of Nitrate to Ammonia by Amidinothiourea-Coordinated Metal Molecular Electrocatalysts with d-π Conjugation.ACS Appl Mater Interfaces. 2024 Oct 16;16(41):54951-54959. doi: 10.1021/acsami.4c11747. Epub 2024 Oct 4. ACS Appl Mater Interfaces. 2024. PMID: 39365186 Review.
-
Single Atom Catalyst for Nitrate-to-Ammonia Electrochemistry.Small. 2025 Jul;21(28):e2403515. doi: 10.1002/smll.202403515. Epub 2024 Sep 30. Small. 2025. PMID: 39350454 Free PMC article. Review.
References
-
- Costa G. F., Nagao R.. Efficient nitrate-to-ammonia conversion for circular nitrogen economy. Chem. Catal. 2024;4:101193. doi: 10.1016/j.checat.2024.101193. - DOI
-
- Shi Y., Li H., Liu X., Zhang X., Zhan G., Cheng J., Wang J., Mao C., Zhang L.. Green energy-driven ammonia production for sustainable development goals. Chem. 2024;10:2636–2650. doi: 10.1016/j.chempr.2024.06.014. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
