Primary central nervous system lymphoma: Predictors of response to induction therapy and patterns of progression
- PMID: 40575413
- PMCID: PMC12199336
- DOI: 10.1093/noajnl/vdaf082
Primary central nervous system lymphoma: Predictors of response to induction therapy and patterns of progression
Abstract
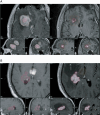
Background: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive variant of non-Hodgkin lymphoma. While PCNSL is often sensitive to induction high-dose methotrexate (HDMTX) based chemotherapy, recurrence rates remain high, approaching 50% within 5 years. The most common molecular alterations in PCNSL include mutations in MYD88 and CD79 and CDKN2A homozygous deletion. There are no predictive or prognostic molecular markers in PCNSL.
Methods: We conducted a retrospective review of 40 patients with PCNSL treated at Thomas Jefferson University and Ohio State University between 2011 and 2023. We created a clinically annotated database of patient characteristics and outcomes. For 13 patients whose paraffin-embedded tissue was available for analysis, Illumina's Infinium Global Diversity Array with Cytogenetics was used to make copy number change calls.
Results: The most commonly used induction chemotherapy regimens were HDMTX monotherapy and HDMTX with rituximab. The overall response rate to induction chemotherapy was 75%. A total of 25% had resistant disease to induction chemotherapy. The median follow-up was 20.3 months. The median progression-free survival for the entire cohort was 30.64 months (range 3.42-57.86 months); 2.56 months for the resistant group and 44.88 months for the sensitive group (P-value < .001). The median overall survival for the entire cohort was 64.8 months (range 41.47-88.13 months); 13.97 months for the resistant group and 81.43 months for the sensitive group (P-value = .046).
Conclusions: The initial response to induction chemotherapy is an important prognostic factor in PCNSL. There is a need for improved predictive biomarkers of response to treatment in PNCLS.
Keywords: CDKN2A; HDMTX; PCNSL; induction chemotherapy; relapse.
© The Author(s) 2025. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
Louis Cappelli: none declared. Allison Kayne: none declared. Jennifer Newman: none. 243 declared. Ahmed Elguindy: none declared. Narendranath Epperla: none declared. Joshua D. Palmer:. 244 grants from Genetech (Phase II clinical trial in HCRN network, outside submitted work), NIH R702. 245 (Photon vs Proton clinical study, outside submitted work), NIH R01CA269948 (Multi-institutional GBM. 246 imaging Artificial Intelligence study, outside submitted work), payments/honoraria from Varian Medical. 247 Systems, Novocure, and ICOTEC. Ubaldo Martinez Outschoorn: none declared. Pierluigi Porcu: none. 248 declared. Wenyin Shi: consulting fees from BrainLab, Novocure, Zai Lab, and Varian Medical System. Iyad. 249 Alnahhas: none declared.
Figures
References
-
- Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE.. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570–4574. - PubMed
-
- DeAngelis LM, Yahalom J, Thaler HT, Kher U.. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635–643. - PubMed
-
- Ferreri AJ, Reni M, Foppoli M, et al. ; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
