Metal halide perovskites for energy applications: recent advances, challenges, and future perspectives
- PMID: 40575558
- PMCID: PMC12199149
- DOI: 10.1039/d5ra02730f
Metal halide perovskites for energy applications: recent advances, challenges, and future perspectives
Abstract
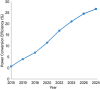
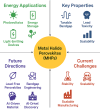
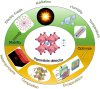
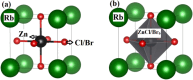
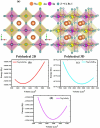
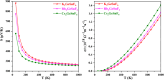
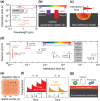
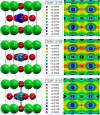
Metal halide perovskites (MHPs) have rapidly emerged as a leading class of materials for a wide range of energy applications, including photovoltaics, light-emitting devices, and energy storage systems. Their exceptional optoelectronic properties such as high absorption coefficients, long carrier diffusion lengths, and tunable bandgaps combined with their low-cost, solution-processable synthesis methods, position MHPs at the forefront of next-generation sustainable energy technologies. Despite these advantages, critical challenges remain, particularly concerning their long-term operational stability, environmental toxicity (especially due to the lead content), and scalability for industrial production. This review comprehensively examines recent progress in the synthesis and characterization of MHPs, focusing on key breakthroughs in materials design, processing techniques, and analytical tools that deepen our understanding of their structure property performance relationships. We also discuss the primary bottlenecks limiting commercial deployment and highlight emerging strategies to improve device durability, reduce ecological impact, and enhance compatibility with scalable manufacturing processes. Finally, we offer a forward-looking perspective on promising research directions aimed at expanding the applicability of MHPs beyond photovoltaics, including their potential roles in thermoelectric conversion, solid-state batteries, and advanced optoelectronic sensors, thereby underscoring their transformative potential in the future of clean energy technologies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest related to this work.
Figures




References
-
- Wang C. et al., On-demand airport slot management: tree-structured capacity profile and coadapted fire-break setting and slot allocation. Transp. A: Transp. Sci. 2024:1–35.
-
- Ye D. et al., PO-SRPP: A decentralized pivoting path planning method for self-reconfigurable satellites. IEEE Trans. Ind. Electron. 2024;71(11):14318–14327.
-
- Xiao Y. Yang Y. Ye D. Zhang J. Quantitative precision second-order temporal transformation-based pose control for spacecraft proximity operations. IEEE Trans. Aero. Electron. Syst. 2025;61(2):1931–1941.
-
- Xiao Y. Yang Y. Ye D. Zhao Y. Scaling-transformation based attitude tracking control for rigid spacecraft with prescribed time and prescribed bound. IEEE Trans. Aero. Electron. Syst. 2025;61(1):433–442.
-
- Zhang H. H. et al., 5G base station antenna array with heatsink radome. IEEE Trans. Antenn. Propag. 2024;72(3):2270–2278.
Publication types
LinkOut - more resources
Full Text Sources

