Silk fibroin hydrogel with recombinant silk fibroin/NT3 protein enhances wound healing by promoting type III collagen synthesis and hair follicle regeneration in skin injury
- PMID: 40575657
- PMCID: PMC12198047
- DOI: 10.1016/j.mtbio.2025.101957
Silk fibroin hydrogel with recombinant silk fibroin/NT3 protein enhances wound healing by promoting type III collagen synthesis and hair follicle regeneration in skin injury
Abstract
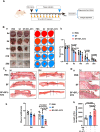
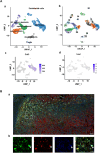
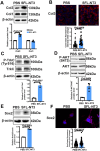
Scar formation on skin wounds remains a considerable challenge in regenerative medicine. Various wound dressings, composed of biomaterials alone or in combination with bioactive factors, have been developed to improve healing outcomes. In this study, we designed a recombinant neurotrophin-3 (NT3) containing a silk fibroin light chain (SFL) and developed a silk fibroin (SF) hydrogel with NT3 activity. The SFL-NT3 protein bound to the heavy-light chains of SF and was efficiently integrated into the SF hydrogel. We evaluated the effect of the recombinant NT3-SFL hydrogel on wound healing in a mouse skin injury model. This hydrogel enhanced wound healing. Remarkably, SFL-NT3 increased the levels of type III collagen (Col3) during the healing process and induced hair follicle formation, which is a characteristic of scar-less healing. Further investigation revealed that SFL-NT3 upregulated Col3 expression in skin fibroblasts expressing the NT3 receptor, TrkC. NT3 activation of TrkC leads to Akt phosphorylation, resulting in elevated Sox2 levels, which in turn enhances Col3 transcription. Notably, TrkC inhibition abrogated the beneficial effects of SF + SFL-NT3 on wound healing, confirming its involvement in this signaling pathway. In conclusion, the SF hydrogel loaded with SFL-NT3 facilitated rapid and reduced scarring during wound healing, providing a promising approach for the clinical treatment of SF-based biomaterials that incorporate bioactive factors.
Keywords: Neurotrophin-3; Scar; Silk fibroin hydrogel; Skin injury; Type III collagen.
© 2025 The Authors.
Conflict of interest statement
The authors have no competing interests to declare.
Figures






References
LinkOut - more resources
Full Text Sources

