Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- PMID: 40575730
- PMCID: PMC12198943
- DOI: 10.1515/biol-2022-1032
Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
Abstract
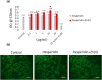
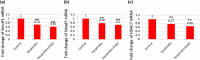
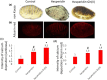
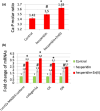
This investigation explores the impact of hesperidin and its zinc(ii) complex on osteoblast differentiation and subsequent bone formation. The biocompatibility of synthesized complexes (0-20 μg/mL) was assessed in vitro using mouse mesenchymal stem cells, while in vivo toxicity was evaluated using a chick embryo model. Both hesperidin and its zinc(ii) complex were found to be non-toxic at a concentration of 10 μg/mL. Notably, these compounds significantly increased alkaline phosphatase activity and enhanced calcium deposition. Molecular analyses revealed upregulation of Runx2 and type 1 collagen mRNA expression, along with increased levels of osteonectin and osteocalcin proteins, while negative regulators of osteoblast differentiation (Smad7, Smurf1, HDAC7) were downregulated. A new aspect of this study is demonstrating that the zinc(ii) complex of hesperidin uniquely enhances osteogenic activity compared to hesperidin alone, highlighting its potential to improve bone formation significantly. Additionally, we elucidated the role of miR-143-3p in mediating these effects, achieved through HDAC7 suppression and enhanced Runx2 expression, assessed using the pmirGLO dual luciferase reporter system. Zebrafish studies further demonstrated the complexes' effects on bone formation, revealing increased osteoblastic activity and improved calcium-to-phosphorus ratios in regenerated scales. These findings underscore the potential of hesperidin-Zn(ii) as a promising therapeutic agent for bone tissue engineering.
Keywords: bone; hesperidin; microRNA; osteoblast; zebrafish; zinc(ii).
© 2025 the author(s), published by De Gruyter.
Conflict of interest statement
Conflict of interest: Authors state no conflict of interest.
Figures




References
-
- Abdelgawad AAM, El Bassossy TAI, Ahmed FA. Synthesis, chemical characterization, and biological investigation of naturally isolated hesperidin and its metal complexes. Bull Chem Soc Ethiop. 2024;38(2):385–95. 10.4314/bcse.v38i2.8. - DOI
-
- Binkowska I. Hesperidin: synthesis and characterization of bioflavonoid complex. SN Appl Sci. 2020;2:445. 10.1007/s42452-020-2256-8. - DOI
-
- Chen B, Zhu S. Spectroscopic and structural study of the zinc(II)-hesperidin complexes and its analysis application. J Chem Soc Pak. 2018;40(4):682–9.
LinkOut - more resources
Full Text Sources
