Longitudinal Assessment of 4-Year HFMSE Changes in SMA II and III Patients Treated With Nusinersen
- PMID: 40576143
- PMCID: PMC12203395
- DOI: 10.1111/ene.70268
Longitudinal Assessment of 4-Year HFMSE Changes in SMA II and III Patients Treated With Nusinersen
Abstract
Background: The aim of this international retrospective study was to assess 4-year change using the Hammersmith Functional Motor Scale Expanded (HFMSE) in individuals with type II and III spinal muscular atrophy (SMA) treated with nusinersen and to establish predictors of HFMSE changes.
Methods: Individuals with type II or III SMA, and at least 4 years of nusinersen-only treatment were included. All were assessed using the HFMSE. Age at baseline, sex, motor function, SMN2 copy number, and age of onset were also retrospectively collected. Linear mixed effect models were used to calculate yearly changes and trajectory predictors.
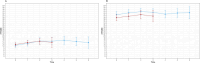
Results: We included 73 individuals with SMA type II (mean age 8.58 years, SD 7.91, IQR 3.04-10.70) and 111 type III (mean age 7.91 years, SD 17.83, IQR 8.15-34.42). Over 4 years, mean changes were + 4.18 (95% CI: 2.85-5.50) for SMA II and + 1.08 (95% CI: 0.12-2.04) for SMA III. Age (SMA II: -0.34\[-0.51 to -0.17]; SMA III: -0.13\[-0.20 to -0.06], p < 0.001) and baseline HFMSE (SMA II: 1.02\[0.70-1.34]; SMA III: 0.79\[0.71-0.87], p < 0.001) were the strongest predictors of progression, with younger age and higher baseline scores associated with better outcomes. Functional status was only predictive for type III (6.96\[4.26-9.66]).
Conclusion: Our results confirm that, given a follow up of 4 years, there is a persistent impact of nusinersen on clinical progression that is better observed in younger patients with higher HFMSE scores at baseline, especially during the first 2 years of treatment.
Keywords: Hammersmith functional motor scale expanded; long term results; motor function; nusinersen; spinal muscular atrophy.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Coratti G, Pane M, Pasternak A, Albamonte E, Pera MC, Glanzman A, Montes J, De Sanctis R, Duong T, Dunaway Young S, Civitello M, Sansone AV, D'Amico A, Bruno C, Messina S, Bertini E, Day J, Ricci F, Mongini T, Finkel R, and Mercuri E report personal fees for advisory boards, steering committees, speaker fees, or consultancies from BIOGEN S.R.L., ROCHE, AVEXIS and/or NOVARTIS outside the submitted work. Zolkipli‐Cunningham Z reports support from CURE SMA outside the submitted work. Bovis F, Rohwer A, Darras BT, Hirano M, Sframeli M, Catteruccia M, Mizzoni I, Rolle E, Bravetti C, Cavallina I, Morando S, Brolatti N, and Salmin F have nothing to disclose.
Figures

References
-
- Dubowitz V., “Chaos in Classification of the Spinal Muscular Atrophies of Childhood,” Neuromuscular Disorders 1 (1991): 77–80. - PubMed
-
- Audic F., Dubois S. M., Durigneux J., et al., “Effect of Nusinersen After 3 Years of Treatment in 57 Young Children With SMA in Terms of SMN2 Copy Number or Type,” Archives de Pédiatrie 31 (2024): 117–123. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

