Cost-effectiveness analysis of routine pediatric vaccination of 15-valent pneumococcal conjugate vaccine in South Korea
- PMID: 40576170
- PMCID: PMC12218592
- DOI: 10.1080/21645515.2025.2515650
Cost-effectiveness analysis of routine pediatric vaccination of 15-valent pneumococcal conjugate vaccine in South Korea
Abstract
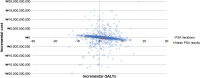
Pneumococcal disease poses a substantial clinical and economic burden, especially among children under 5 years old. The 13-valent pneumococcal conjugate vaccine (PCV13) was first introduced in South Korea's Childhood Immunization Program in 2014. In October 2023, the Ministry of Food and Drug Safety approved the use of a 15-valent pneumococcal conjugate vaccine (PCV15) in infants, children, adolescents, and adults. The aim of this study was to evaluate the cost savings of routine vaccination with PCV15 versus PCV13 in a pediatric population in South Korea. A Markov model was adapted to estimate costs and health outcomes from a societal perspective over a 100-year time horizon. The model estimated the impact of PCV15 versus PCV13 on pneumococcal disease incidence, post-meningitis sequalae, and deaths. The effectiveness of PCV15 was extrapolated from PCV13 data. Herd immunity effects were applied. Costs and epidemiological data were obtained from published literature and the National Health Information Database. Costs were reported in 2023 Korean Won (₩) with USD ($) equivalents. Quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios were estimated. The model assumed a discount rate of 4.5%. PCV15 was projected to provide a total savings of ₩36,213,756 [$27,751] with a gain of 2 QALYs versus PCV13. Under the model assumptions, switching from PCV13 to PCV15 is a cost-saving option for South Korea's routine pediatric pneumococcal vaccination program.
Keywords: Invasive pneumococcal disease; Streptococcus pneumoniae; acute otitis media; bacteremic pneumococcal pneumonia.
Conflict of interest statement
Min Huang and Salini Mohanty are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Young June Choe works with Department of Pediatrics, Korea University College of Medicine, Seoul, South Korea. Jihyun Park, Yunjin Jeon, Gyongseon Yang, and Hyesun Kim are employees of MSD Korea, Seoul, South Korea, Isaya Sukarom and Lefteris Floros are employees of MSD Thailand, Bangkok, Thailand and MSD Switzerland, Zurich, Switzerland respectively. Nitika Chhabra, Shalini Kumari and Jasmeet Singh are employees of CHEORS, North Wales, PA, USA.
Figures
References
-
- Centers for Disease Control and Prevention . Pneumococcal disease (Streptococcus pneumoniae). CDC. ; [accessed. 2019. May]. https://wwwnc.cdc.gov/travel/diseases/pneumococcal-disease-streptococcus....
-
- Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018. June; 17(6):479–493. doi: 10.1080/14760584.2018.1413354. - DOI - PubMed
-
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, McCarthy ND, Petrou S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Global Health. 2017. Jan; 5(1):e51–e59. doi: 10.1016/s2214-109x(16)30306-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical