14-3-3ε inhibits premature centriole disengagement by inhibiting the activity of Plk1 and separase
- PMID: 40576425
- PMCID: PMC12301659
- DOI: 10.1242/jcs.263808
14-3-3ε inhibits premature centriole disengagement by inhibiting the activity of Plk1 and separase
Abstract
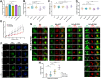
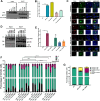
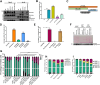
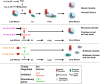
The 14-3-3 protein family regulates several pathways in mammalian cells, including centrosome duplication. However, the precise mechanisms by which 14-3-3 paralogs regulate the centrosome cycle remain unclear. To identify the mechanisms by which 14-3-3ε regulates centrosome duplication, we altered two conserved acidic residues in the 14-3-3ε phospho-peptide-binding pocket that regulate complex formation and dissociation with the associated ligands, D127 and E134, to alanine. Altering these residues to alanine led to opposing effects on centrosome duplication; the D127A mutant inhibited centrosome duplication, whereas cells expressing the E134A mutant showed the presence of supernumerary centrosomes. We demonstrate that 14-3-3ε does not inhibit centriole duplication, as reported for 14-3-3γ, but inhibits centriole disengagement. Using a combination of pharmacological and genetic approaches, we demonstrate that 14-3-3ε inhibits the activity of Plk1 and separase [also known as separin (ESPL1)], leading to disengagement defects that ultimately lead to decreased proliferation and cell death. Our work demonstrates that different 14-3-3 paralogs regulate different steps in the centrosome cycle and that disrupting complex formation between 14-3-3ε and Plk1 or separase could be a novel therapeutic strategy in tumor cells.
Keywords: 14-3-3ε; Centriole disengagement; Centrosome cycle; Plk1; Separase.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
- BT/PR38272/BRB/10/1894/2020/Department of Biotechnology, Government of India
- 1/3(7)/2020/TMC/R&D-II/ 8823 and 1/3(6)/2020/TMC/R&D -II/ 3805/Department of Atomic Energy, Government of India
- 4338/Advanced Centre for Treatment, Research and Education in Cancer
- BT/PR38272/BRB/10/1894/2020/Department of Biotechnology
- 1/3(7)/2020/TMC/R&D-II/8823/Department of Atomic Energy
LinkOut - more resources
Full Text Sources
Miscellaneous

