Canine Adrenomedullary and Pheochromocytoma Organoids: A Novel In Vitro Model
- PMID: 40576438
- PMCID: PMC12264429
- DOI: 10.1210/endocr/bqaf114
Canine Adrenomedullary and Pheochromocytoma Organoids: A Novel In Vitro Model
Abstract
Context: Given the lack of effective medical treatment for pheochromocytomas (PCCs), a reliable in vitro model is needed to explore new therapies. Organoids are three-dimensional (3D) self-renewing structures that exhibit key features of their tissue of origin, providing valuable platforms for disease modeling and drug screening.
Objective: This study aimed to establish and characterize organoid cultures of canine normal adrenal medullas and PCCs.
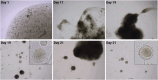
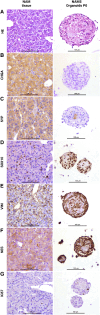
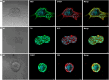
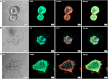
Methods: Normal adrenal medullas from healthy dogs and tumor tissue from client-owned dogs with PCC were used to develop organoids. Primary cell suspensions were cultured in a 3D matrix, and organoids were established under optimized conditions. Organoids were characterized using histology, immunohistochemistry, immunofluorescence, qPCR, and metanephrine analysis by LC-MS/MS.
Results: Five adrenomedullary organoid lines were successfully established, demonstrating sustained growth. Organoid cultures were also derived from 9 PCCs, although expansion was limited after passages 1 to 2. Both adrenomedullary and PCC organoids expressed differentiation markers (chromogranin A, synaptophysin, phenylethanolamine N-methyltransferase) and stem/progenitor markers (nestin, SOX10). Organoids retained key functional traits, as indicated by metanephrine levels in culture supernatants, which initially mirrored primary tumor patterns. A decline in both differentiation marker expression and metanephrine levels was observed over time, possibly due to organoid dedifferentiation or selective loss of differentiated chromaffin cells.
Conclusion: This study demonstrates the establishment of the first adrenomedullary and PCC organoid lines. While further optimization is needed, these organoids offer valuable potential as an in vitro model to investigate PCC pathophysiology and explore novel treatment strategies for this therapeutically challenging tumor.
Keywords: adrenal; adrenal medulla; culture; dog; modeling.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures










Similar articles
-
Organoid Models Established from Primary Tumors and Patient-Derived Xenograft Tumors Reflect Platinum Sensitivity of Ovarian Cancer Patients.bioRxiv [Preprint]. 2025 May 2:2024.06.28.601283. doi: 10.1101/2024.06.28.601283. bioRxiv. 2025. PMID: 40654830 Free PMC article. Preprint.
-
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling.Bio Protoc. 2025 Jun 20;15(12):e5354. doi: 10.21769/BioProtoc.5354. eCollection 2025 Jun 20. Bio Protoc. 2025. PMID: 40620811 Free PMC article.
-
Mesenchymal stem/stromal cells from human pluripotent stem cell-derived brain organoid enhance the ex vivo expansion and maintenance of hematopoietic stem/progenitor cells.Stem Cell Res Ther. 2024 Mar 5;15(1):68. doi: 10.1186/s13287-023-03624-w. Stem Cell Res Ther. 2024. PMID: 38443990 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Ethical considerations for advancing research using organoid models derived from the placenta.Hum Reprod Update. 2025 Jul 1;31(4):392-401. doi: 10.1093/humupd/dmaf007. Hum Reprod Update. 2025. PMID: 40096642 Free PMC article. Review.
References
-
- Lenders JWM, Kerstens MN, Amar L, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38(8):1443‐1456. - PMC - PubMed
-
- Bayley JP, Devilee P. Advances in paraganglioma-pheochromocytoma cell lines and xenografts. Endocr Relat Cancer. 2020;27(12):R433‐R450. - PubMed
-
- Tischler AS, Favier J. Progress and challenges in experimental models for pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2023;30(5):e220405. - PubMed
-
- Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-PronO study): a European network for the study of adrenal tumors retrospective study. J Clin Endocrinol Metab. 2019;104(6):2367‐2374. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

