Comparative analysis of brain-derived tau oligomer interactomes in Alzheimer's disease, non-demented with Alzheimer's neuropathology, and primary age-related tauopathy: Implications for neurodegeneration and cognitive resilience
- PMID: 40576462
- PMCID: PMC12322345
- DOI: 10.1177/13872877251352382
Comparative analysis of brain-derived tau oligomer interactomes in Alzheimer's disease, non-demented with Alzheimer's neuropathology, and primary age-related tauopathy: Implications for neurodegeneration and cognitive resilience
Abstract
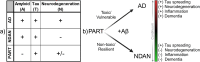
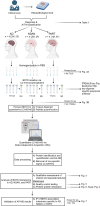
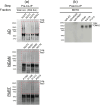
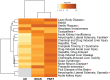
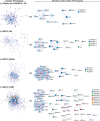
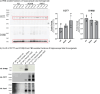
BackgroundIn Alzheimer's disease (AD), soluble tau oligomers are central to neurodegeneration and cognitive decline. Resilient individuals, such as those with non-demented Alzheimer's neuropathology (NDAN) or primary age-related tauopathy (PART), offer critical insights into protective mechanisms against tau-mediated neurodegeneration. NDAN individuals exhibit AD neuropathology without cognitive impairment or neurodegeneration, while PART, characterized by hippocampal- and entorhinal-restricted tau pathology, manifests with minimal-to-no amnestic changes. Brain-derived tau oligomers (BDTO) from these cohorts provide a unique platform to explore molecular pathways underlying both vulnerability and resilience to tau pathology.ObjectiveTo identify vulnerability- and resilience-associated pathways by comparing BDTO interactomes across AD, NDAN, and PART.MethodsBDTO were isolated from AD (n = 4; 2M, 2F), NDAN (n = 4; 2M, 2F), and PART (n = 4; 1M, 3F) hippocampal autopsy specimens using co-immunoprecipitation. Proteins were identified via liquid chromatography-tandem mass spectrometry, and non-specific interactors were filtered using SAINTq. Interactome networks and enrichment analyses were performed using Metascape. Findings were cross-referenced with the Neuropro database and existing literature on tangle-associated proteins. Key interactors were validated through reverse co-immunoprecipitation.ResultsA total of 203 proteins were identified, including eight novel interactors not previously linked to AD. All interactomes were enriched in proteins related to tau physiology and lysosomal degradation. NDAN and PART interactomes showed unique enrichment in proteins involved in cellular responses to reactive oxygen species.ConclusionsOne vulnerability-associated and 18 resilience-associated pathways that may mitigate tau-mediated neurodegeneration were identified, laying the groundwork for novel diagnostic and therapeutic strategies targeting pathological tau oligomers.
Keywords: Alzheimer's disease; cognitive dysfunction; hippocampus; tandem mass spectrometry; tauopathies.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
A brain-derived tau oligomer polymorph is associated with cognitive resilience to Alzheimer's disease.Alzheimers Dement. 2025 Aug;21(8):e70550. doi: 10.1002/alz.70550. Alzheimers Dement. 2025. PMID: 40823851 Free PMC article.
-
Amyloid-β oligomers increase the binding and internalization of tau oligomers in human synapses.Acta Neuropathol. 2024 Dec 17;149(1):2. doi: 10.1007/s00401-024-02839-2. Acta Neuropathol. 2024. PMID: 39688618 Free PMC article.
-
Selective vulnerability and resilience to Alzheimer's disease tauopathy as a function of genes and the connectome.Brain. 2025 Jul 9:awaf179. doi: 10.1093/brain/awaf179. Online ahead of print. Brain. 2025. PMID: 40631882
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis.Lancet Neurol. 2016 Jun;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3. Epub 2016 Apr 8. Lancet Neurol. 2016. PMID: 27068280
References
-
- Alzheimer's Association. 2024 Alzheimer’s Disease facts and figures. Alzheimer’s Association, https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf (2024). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

