Skeletal Muscle Mitochondria Contain Nuclear-Encoded RNA Species Prior to and Following Adaptation to Exercise Training in Rats
- PMID: 40577041
- PMCID: PMC12204305
- DOI: 10.1096/fj.202500157R
Skeletal Muscle Mitochondria Contain Nuclear-Encoded RNA Species Prior to and Following Adaptation to Exercise Training in Rats
Abstract
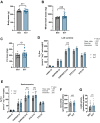
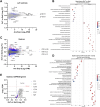
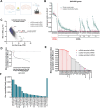
Skeletal muscle mitochondria adaptation to exercise training is mediated by molecular factors that are not fully understood. Mitochondria import over 1000 proteins encoded by the nuclear genome, but the RNA population resident within the organelle is generally thought to be exclusively encoded by the mitochondrial genome. However, recent in vitro evidence suggests that specific nuclear-encoded miRNAs and other noncoding RNAs (ncRNAs) can reside within the mitochondrial matrix. Whether these are present in mitochondria of skeletal muscle tissue, and whether this is affected by endurance training-a potent metabolic stimulus for mitochondrial adaptation-remains unknown. Rats underwent 4 weeks of moderate-intensity treadmill exercise training, then were humanely killed and tissues were collected for molecular profiling. Mitochondria from gastrocnemius skeletal muscle were isolated by immunoprecipitation, further purified, and then the resident RNA was sequenced to assess the mitochondrial transcriptome. Exercise training elicited typical transcriptomic responses and functional adaptations in skeletal muscle, including increased mitochondrial respiratory capacity. We identified 24 nuclear-encoded coding or noncoding RNAs in purified mitochondria, in addition to 50 nuclear-encoded miRNAs that met a specified abundance threshold. Although none were differentially expressed in the exercise vs. control group at FDR < 0.05, exploratory analyses suggested that the abundance of 3 miRNAs was altered (p < 0.05) in mitochondria isolated from trained compared with sedentary skeletal muscle. We report the presence of a specific population of nuclear-encoded RNAs in the mitochondria isolated from rat skeletal muscle tissue, which could play a role in regulating exercise adaptations and mitochondrial biology.
Keywords: exercise; mitochondria; skeletal muscle; transcriptome.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Mitochondria Transplantation: Rescuing Innate Muscle Bioenergetic Impairment in a Model of Aging and Exercise Intolerance.J Strength Cond Res. 2024 Jul 1;38(7):1189-1199. doi: 10.1519/JSC.0000000000004793. J Strength Cond Res. 2024. PMID: 38900170 Free PMC article.
-
Shared and distinct adaptations to early-life exercise training based on inborn fitness.J Physiol. 2025 Jun 15. doi: 10.1113/JP288331. Online ahead of print. J Physiol. 2025. PMID: 40517393
-
PoWeR elicits intracellular signaling, mitochondrial adaptations, and hypertrophy in multiple muscles consistent with endurance and resistance exercise training.J Appl Physiol (1985). 2025 Apr 1;138(4):1034-1049. doi: 10.1152/japplphysiol.00872.2024. Epub 2025 Mar 18. J Appl Physiol (1985). 2025. PMID: 40100208 Free PMC article.
References
-
- Russell A. P., Foletta V. C., Snow R. J., and Wadley G. D., “Skeletal Muscle Mitochondria: A Major Player in Exercise, Health and Disease,” Biochimica et Biophysica Acta 1840 (2014): 1276–1284. - PubMed
-
- Holloszy J. O. and Coyle E. F., “Adaptations of Skeletal Muscle to Endurance Exercise and Their Metabolic Consequences,” Journal of Applied Physiology 56 (1984): 831–838. - PubMed
-
- Holloszy J. O. and Booth F. W., “Biochemical Adaptations to Endurance Exercise in Muscle,” Annual Review of Physiology 38 (1976): 273–291. - PubMed
-
- Spina R. J., Chi M. M., Hopkins M. G., Nemeth P. M., Lowry O. H., and Holloszy J. O., “Mitochondrial Enzymes Increase in Muscle in Response to 7‐10 Days of Cycle Exercise,” Journal of Applied Physiology 80 (1996): 2250–2254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

