Effect of the Multi-Strain Probiotic SYN-53 in the Management of Allergic Rhinoconjunctivitis
- PMID: 40577168
- PMCID: PMC12368763
- DOI: 10.1111/all.16634
Effect of the Multi-Strain Probiotic SYN-53 in the Management of Allergic Rhinoconjunctivitis
Abstract
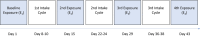
Dysbiosis is increasingly linked to allergy development. This study evaluates the efficacy of the multi-strain probiotic SYN-53 in the management of allergic rhinoconjunctivitis (ARC). Eighty-four subjects with confirmed grass pollen allergy underwent up to three bi-weekly 3-day intake cycles with SYN-53 or placebo. After each cycle, subjects were exposed to grass pollen in an allergen exposure chamber. ARC symptoms were assessed using the Total Symptom Score (TSS) before and after each use of SYN-53. After one intake cycle, SYN-53 already showed a trend towards greater efficacy over placebo, which became significant after two cycles (ΔTSSMAX: -3.44 ± 0.42 vs. -1.87 ± 0.37; p = 0.0067), with 38% vs. 24% symptom relief. In subjects with moderate to severe symptoms, SYN-53 was already significantly superior after one single intake cycle and improved further after two cycles (ΔTSSMAX: -4.78 ± 0.51 vs. -2.43 ± 0.47; p = 0.0014), with 45% vs. 26% symptom relief. SYN-53 is effective in the management of ARC, highlighting the role of bacterial diversity and dosage in probiotic nutritional supplements.
Keywords: allergen exposure chamber; allergic rhinitis; allergic rhinoconjunctivitis; microbiome; probiotic.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The European Centre for Allergy Research Foundation (ECARF) received reimbursement for participating as a study center. K.‐C.B. received honoraria for lectures from ALK, Allergopharma, Almirall, AstraZeneca, Bencard, Berlin‐Chemie, Chiesi, GSK, HAL, Mundipharma, Novartis, Sanofi, and Stallergenes during the last 5 years. T.Z. has received institutional funding for research and/or honoria for lectures and/or consulting from Amgen, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, Blueprint Medicines, FAES, HAL, Henkel, Kryolan, Leti, L'Oreal, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva and UCB, Uriach; in addition, he is a member of ARIA/WHO, DGAKI, ECARF, GA2LEN and WAO.
Figures


Similar articles
-
Carbamylated monomeric allergoids as a therapeutic option for sublingual immunotherapy of dust mite- and grass pollen-induced allergic rhinoconjunctivitis: a systematic review of published trials with a meta-analysis of treatment using Lais® tablets.Acta Dermatovenerol Alp Pannonica Adriat. 2010 Oct;19(3):3-10. Acta Dermatovenerol Alp Pannonica Adriat. 2010. PMID: 20976414
-
Allergen injection immunotherapy for seasonal allergic rhinitis.Cochrane Database Syst Rev. 2007 Jan 24;2007(1):CD001936. doi: 10.1002/14651858.CD001936.pub2. Cochrane Database Syst Rev. 2007. PMID: 17253469 Free PMC article.
-
Sublingual immunotherapy for treating allergic conjunctivitis.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD007685. doi: 10.1002/14651858.CD007685.pub2. Cochrane Database Syst Rev. 2011. PMID: 21735416
-
Sublingual immunotherapy for allergic rhinitis.Cochrane Database Syst Rev. 2003;(2):CD002893. doi: 10.1002/14651858.CD002893. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2010 Dec 08;(12):CD002893. doi: 10.1002/14651858.CD002893.pub2. PMID: 12804442 Updated.
-
Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review.Pediatr Allergy Immunol. 2008 May;19(3):197-207. doi: 10.1111/j.1399-3038.2007.00648.x. Epub 2008 Jan 24. Pediatr Allergy Immunol. 2008. PMID: 18221463
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

