Endothelial metabolic zonation in the vascular network: a spatiotemporal blueprint for angiogenesis
- PMID: 40577232
- PMCID: PMC12315569
- DOI: 10.1152/ajpheart.00352.2025
Endothelial metabolic zonation in the vascular network: a spatiotemporal blueprint for angiogenesis
Abstract
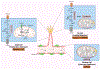
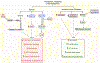
Angiogenesis, a cornerstone of vascular development, tissue regeneration, and tumor progression, is critically orchestrated by the metabolic behavior of endothelial cells (EC). Recent discoveries have redefined EC not as metabolically uniform entities, but as spatially and functionally heterogeneous populations whose metabolic states govern their angiogenic potential. This review presents a comprehensive synthesis of metabolic zonation in EC, spanning arterial, venous, and capillary domains, and highlights cell-type-specific programs during sprouting angiogenesis-including tip, stalk, and phalanx cells. We explore how distinct metabolic pathways-glycolysis, oxidative phosphorylation, fatty acid oxidation, and glutaminolysis-are differentially used across tissue contexts such as the brain, skeletal muscle, kidney, and tumor microenvironments. We discuss technological breakthroughs in spatial metabolomics, temporal (circadian) regulation of endothelial metabolism, and emerging clinical strategies to target EC metabolic vulnerabilities in cancer and ischemic diseases. Furthermore, we advocate for spatiotemporal modeling of EC metabolism using computational and machine learning frameworks to predict angiogenic behavior and accelerate therapeutic discovery. This integrative perspective underscores the need for precision-targeted angiogenic interventions and establishes metabolic zonation as a foundational principle in vascular biology.
Keywords: angiogenesis; circadian rhythm; endothelial cells; metabolic zonation; metabolism.
Conflict of interest statement
Declaration of Competing Interest
None declared.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

