Extreme exercise in males is linked to mTOR signalling and onset of amyotrophic lateral sclerosis
- PMID: 40577240
- PMCID: PMC12493055
- DOI: 10.1093/brain/awaf235
Extreme exercise in males is linked to mTOR signalling and onset of amyotrophic lateral sclerosis
Abstract
Amyotrophic lateral sclerosis (ALS) is thought to be caused by interaction between genetic and environmental factors leading to motor neuron (MN) degeneration. Physical exercise has been linked to ALS but controversy remains. A key question is to determine which individuals might be at risk of exercise-associated ALS, because unnecessary avoidance of exercise could be harmful. We implemented complementary strategies including Mendelian randomization (MR) and multiple questionnaire-based measures of physical exercise in different cohorts. We include a prospective study involving UK Biobank participants where we could test for a relationship between exercise and the timing of future ALS symptom onset. To interrogate the molecular basis of our observations we performed a genetic association study of 'extreme' exercise, equivalent to >6 h of strenuous exercise or >12 h of any leisure-time exercise per week. Our data suggest that the link between increased physical exercise and ALS is particularly important for males who perform the most activity; with no evidence of a link in females. We determined that extreme exercise in males is associated with loss-of-function genetic variants within a number of mammalian target of rapamycin (mTOR) signalling genes that are also differentially expressed in ALS spinal cord. Activity-induced mTOR signalling has been shown to selectively benefit MN. Therefore, our findings could imply that moderate exercise is neuroprotective via enhanced mTOR signalling, but extreme exercise in men is associated with neurotoxicity and ALS via a failure of this mechanism. There was no significant overlap between genes associated with extreme exercise and those associated with ALS risk, consistent with a true gene-environment interaction rather than a shared genetic basis. We are not yet able to make individual-level recommendations regarding exercise and risk of ALS, but our conclusions should provide focus for future investigation.
Keywords: amyotrophic lateral sclerosis (ALS); exercise; gene–environment interaction; mammalian target of rapamycin (mTOR) signalling.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

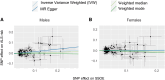
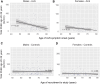
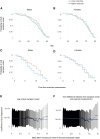

Comment in
-
Unpacking the relationship between exercise and amyotrophic lateral sclerosis.Brain. 2025 Oct 3;148(10):3431-3432. doi: 10.1093/brain/awaf310. Brain. 2025. PMID: 40879079 No abstract available.
References
-
- Zheng X, Wang S, Huang J, et al. Physical activity as risk factor in amyotrophic lateral sclerosis: A systematic review and meta-analysis. J Neurol. 2023;270:2438–2450. - PubMed
-
- Vaage AM, Meyer HE, Landgraff IK, Myrstad M, Holmøy T, Nakken O. Physical activity, fitness, and long-term risk of amyotrophic lateral sclerosis: A prospective cohort study. Neurology. 2024;103:e209575. - PubMed
-
- Shaw PJ, Cooper-Knock J. Physical activity as a risk factor for amyotrophic lateral sclerosis. Neurology. 2024;103:e209689. - PubMed

