Diazotrophic growth of free-living Rhizobium etli: Community-like metabolic modeling of growing and non-growing nitrogen-fixing cells
- PMID: 40577287
- PMCID: PMC12204555
- DOI: 10.1371/journal.pone.0325888
Diazotrophic growth of free-living Rhizobium etli: Community-like metabolic modeling of growing and non-growing nitrogen-fixing cells
Abstract
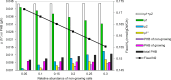
Rhizobium etli, a nitrogen-fixing bacterium, grows both in symbiosis (with plants) and in free-living state. While most metabolic models focus on its symbiotic form, this study refined the existing iOR363 model to account for free-living growth. By addition of a biomass formation reaction followed by model curation growth was simulated using various N-sources (NH₃, NO₂, and NO₃). At fixed succinate uptake rate (4.16 mmol/gDWC/h), ammonia yielded the highest growth rate of 0.259 h ⁻ ¹. To represent free-living N-fixing R. etli, a novel two-member community-like model, consisting of both growing and differentiated non-growing N-fixing cells with ammonia exchange, was developed. The XFBA approach, based on community Flux Balance Analysis (cFBA), was formulated to maintain fixed abundances rather than assuming equal growth rates. With a non-growing:growing abundance ratio of 1:9 in community, N-fixation resulted in lower growth rate of 0.1933 h ⁻ ¹ due to the high energy demand of N₂ assimilation compared to ammonia. Sensitivity analysis revealed that increased abundance of N-fixing cells from 5 to 30% led to decreases of 10% in N2-fixation and 25% in growth rate of growing member. Furthermore, Principal Component Analysis identified oxidative phosphorylation, TCA cycle, and glycolysis as key pathways differentiating flux distributions across N-sources. At high uptake of oxygen, causing nitrogenase inactivity, cytochrome bd oxidase was activated to scavenge oxygen, though at the cost of lower growth rate (by 12% per mmol increase in O2 uptake/gDWC/h). This study provided a platform to obtain insights to free-living state of R. etli which may have applications for other diazotrophs.
Copyright: © 2025 Afarin, Naeimpoor. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Effect of complex microbial preparation of free-living and symbiotic nitrogen-fixing bacteria for agricultural crops.Braz J Biol. 2025 Jul 28;85:e292171. doi: 10.1590/1519-6984.292171. eCollection 2025. Braz J Biol. 2025. PMID: 40736111
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Cummings S, Humphry D, Santos S, Andrews M, James E. The potential and pitfalls of exploiting nitrogen fixing bacteria in agricultural soils as a substitute for inorganic fertiliser. Environ Biotechnol. 2006;2(1):1–10.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

