Randomized Open-Label Clinical Trial Comparing Prednisolone and Cyclosporine With a Nonrandomized Active Control for Treating Presumed Chronic Pancreatitis in Cats
- PMID: 40577334
- PMCID: PMC12204215
- DOI: 10.1111/jvim.70163
Randomized Open-Label Clinical Trial Comparing Prednisolone and Cyclosporine With a Nonrandomized Active Control for Treating Presumed Chronic Pancreatitis in Cats
Abstract
Background: Current management for chronic pancreatitis in cats is largely symptomatic. Anecdotal reports suggest that immunomodulatory treatment can be helpful in some cases, but limited data is available.
Objectives: Compare the effects of symptomatic treatments alone, an immunosuppressive dosage of prednisolone, or modified cyclosporine on serum feline pancreatic lipase immunoreactivity (fPLI) concentration and clinical activity index (CAI).
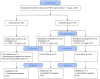
Animals: Forty-eight client-owned cats with a presumptive diagnosis of chronic pancreatitis were managed on an outpatient basis.
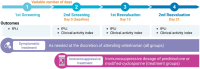
Methods: Three-week randomized open-label trial with a nonrandomized active control. Owners elected to join either the control or the treatment group; cats enrolled in the treatment group were randomized to receive either prednisolone or cyclosporine. Serum fPLI concentration and clinical signs were recorded at baseline and on Days 10 and 21.
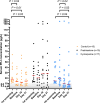
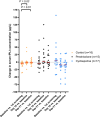
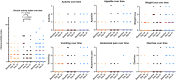
Results: The average decrease in serum fPLI concentration was 13.0 μg/L (95% CI, -23.9 to -0.9 μg/L) larger for the cyclosporine group (n = 17) than for the control group (n = 16) and 27.6 μg/L (95% CI, -41.2 to -11.4 μg/L) larger than for the prednisolone group (n = 15). The average decrease in CAI was 1.9 points (95% CI, -2.7 to -1.2) larger for the prednisolone group than for the control group and 1.2 points (95% CI, -2.1 to -0.4) larger than for the cyclosporine group.
Conclusions: Over a 3-week treatment period, cats with presumed chronic pancreatitis that received cyclosporine had a larger decrease in serum fPLI concentration compared with cats that were treated with an immunosuppressive dosage of prednisolone or cats that received only symptomatic treatments. However, clinical improvement was more apparent with prednisolone, but not cyclosporine.
Keywords: CsA; Spec fPL; corticosteroid; feline; hyperlipasemia; pancreas; pancreatic lipase.
© 2025 The Author(s). Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Drs. Yu‐An Wu, Jonathan A. Lidbury, and Jörg M. Steiner are affiliated with the Gastrointestinal Laboratory at Texas A&M University, which offers laboratory testing, including measurement of serum fPLI concentration, on a fee‐for‐service basis. Dr. Jörg M. Steiner is also a paid consultant for IDEXX Laboratories. Both Drs. Jonathan A. Lidbury and Jörg M. Steiner have acted as paid speakers for IDEXX Laboratories. Dr. Samiran Sinha declares no conflicts of interest.
Figures
References
-
- De Cock H. E., Forman M. A., Farver T. B., and Marks S. L., “Prevalence and Histopathologic Characteristics of Pancreatitis in Cats,” Veterinary Pathology 44, no. 1 (2007): 39–49. - PubMed
-
- Sakai M., Harada K., Matsumura H., et al., “A Case of Feline Pancreatitis,” Journal of Veterinary Medical Science 68, no. 12 (2006): 1331–1333. - PubMed
-
- Ruaux C. G., Steiner J. M., and Williams D. A., “Relationships Between Low Serum Cobalamin Concentrations and Methlymalonic Acidemia in Cats,” Journal of Veterinary Internal Medicine 23, no. 3 (2009): 472–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
