Proteomic and phosphoproteomic analysis of rabies pathogenesis in the clinical canine brain and identification of a kinase inhibitor as a potential repurposed antiviral agent
- PMID: 40577348
- PMCID: PMC12204518
- DOI: 10.1371/journal.pone.0323931
Proteomic and phosphoproteomic analysis of rabies pathogenesis in the clinical canine brain and identification of a kinase inhibitor as a potential repurposed antiviral agent
Abstract
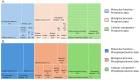
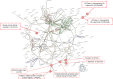
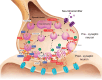
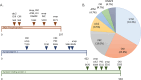
Rabies is a fatal zoonosis caused by the rabies virus (RABV) that has afflicted humans for thousands of years. RABV infection leads to neurological symptoms and death; however, its pathogenesis in the brain is unclear, which complicates patient care. Given that no treatment exists for symptomatic cases, there is an urgent need for effective antiviral drugs. In this study, we aimed to investigate the pathogenic mechanism of RABV in the brain and screen for potential anti-RABV drugs. Protein samples were extracted from the brains of RABV-positive and RABV-negative dogs, and proteomic and phosphoproteomic analyses were conducted. The results showed that the synaptic vesicle cycle is critical to RABV pathogenesis. The kinases involved in the phosphorylation of proteins in the synaptic vesicle cycle were identified and examined as potential drug targets. Casein kinase 2 and protein kinase C were found to be key kinases for RABV replication, and five inhibitors of these enzymes were tested for their anti-RABV properties. Pretreating cells with the kinase inhibitor sunitinib significantly reduced the viral yield after RABV infection. Our findings suggest that RABV interferes with synaptic communication, which leads to rabies, and that inhibiting a vital kinase can reduce viral production. Hence, our findings have implications for the development of rabies treatment regimes.
Copyright: © 2025 Chienwichai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Baker HJ, Martin DR, Gross AL, Chamorro MF, Naskou MC, Johnson AK, et al. Rabies: who should care? J Am Vet Med Assoc. 2022;261(4):592–6. - PubMed
-
- Mitrabhakdi E, Shuangshoti S, Wannakrairot P, Lewis RA, Susuki K, Laothamatas J. Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. J Neurol Sci. 2005;238(1–2):3–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

