Large-scale experimental assessment of variant effects on the structure and function of the citrate transporter SLC13A5
- PMID: 40577459
- PMCID: PMC12204159
- DOI: 10.1126/sciadv.adx3011
Large-scale experimental assessment of variant effects on the structure and function of the citrate transporter SLC13A5
Abstract
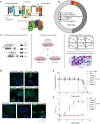
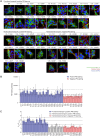
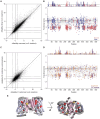
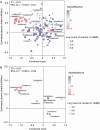
Citrate is an essential metabolite playing critical roles in metabolism and as a neuromodulator during cellular differentiation and development. Mutations in the human citrate transporter SLC13A5, highly expressed in neurons, have been associated with a debilitating form of epileptic encephalopathy. In this study, we used deep mutational scanning to reveal the effect of 90% of all possible single missense variants on the structure and function of SLC13A5. Computational analyses and a detailed experimental validation of 38 variants revealed an accuracy of 86% and provided mechanistic interpretations for deleterious mutations, including the effect on protein stability, trafficking, and citrate transport. Analyses of blood citrate concentration from individuals enrolled in the UK Biobank study supported our analyses. The results illustrate an unbiased mutational landscape of the citrate transporter, illuminate mechanisms of pathogenicity, and offer a platform for the analysis of specific variants as well as opportunities for the future development of intervention strategies.
Figures

Similar articles
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Mental Health First Aid as a tool for improving mental health and well-being.Cochrane Database Syst Rev. 2023 Aug 22;8(8):CD013127. doi: 10.1002/14651858.CD013127.pub2. Cochrane Database Syst Rev. 2023. PMID: 37606172 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
-
- Tannahill G. M., Curtis A. M., Adamik J., Palsson-McDermott E. M., McGettrick A. F., Goel G., Frezza C., Bernard N. J., Kelly B., Foley N. H., Zheng L., Gardet A., Tong Z., Jany S. S., Corr S. C., Haneklaus M., Caffrey B. E., Pierce K., Walmsley S., Beasley F. C., Cummins E., Nizet V., Whyte M., Taylor C. T., Lin H., Masters S. L., Gottlieb E., Kelly V. P., Clish C., Auron P. E., Xavier R. J., O’Neill L. A., Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). - PMC - PubMed
-
- Petroff O. A., Yu R. K., Ogino T., High-resolution proton magnetic resonance analysis of human cerebrospinal fluid. J. Neurochem. 47, 1270–1276 (1986). - PubMed
-
- D. T. Monaghan, D. E. Jane, “Pharmacology of NMDA receptors” in Biology of the NMDA Receptor, A. M. Van Dongen, Ed. (Taylor & Francis, 2009), pp. 257–282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

